Preparation and application of targeted anti-tumor fusion protein lpo
A fusion protein and plasmid technology, applied in the direction of anti-tumor drugs, hybrid peptides, drug combinations, etc., can solve the problem of low tumor suppression efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
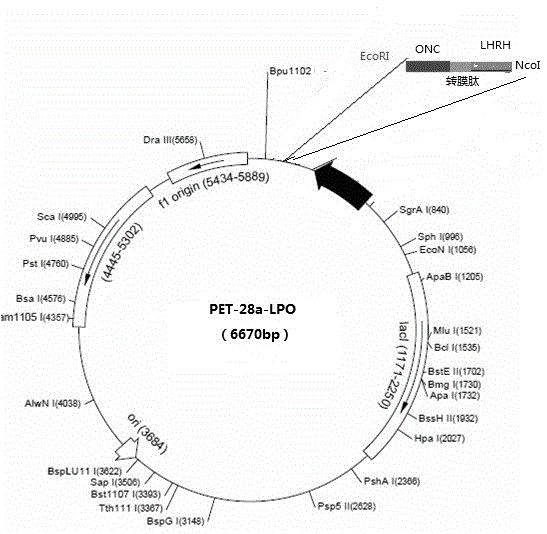
[0053] Embodiment 1: the preparation of LPO expression vector and engineering bacterium
[0054] Construction and Identification of Recombinant Expression Plasmids
[0055] a. Gene synthesis: In the present invention, according to the above-mentioned design concept, the design of the restriction site and the whole gene sequence of LPO is carried out, and the following sequence is artificially synthesized in vitro:
[0056] NcoI endonuclease recognition sequence (CCATGG)+GC+LHRH polypeptide gene+PEA transmembrane peptide+ONC sequence+stop codon (TAA)+EcoRI endonuclease recognition sequence (GAATTC), see SEQ ID NO: 1 for the gene sequence, this synthesis The sequence was directly cloned into the T vector provided by Dalian Takara Company, and transformed into Escherichia coli JM109 bacteria. The positive clones were identified by PCR, and the positive clones were expanded and cultivated. The plasmid DNA was extracted by conventional molecular cloning techniques, and digested wit...
Embodiment 2
[0059] Example 2: Expression of LPO fusion protein and purification of product:
[0060] Escherichia coli BL21 (DE3) (containing T 7 RNA polymerase gene) were cultured on LB agar plates containing kanamycin (50 μg / ml). After culturing, select kanamycin-resistant colonies and culture them in LB medium containing kanamycin (50 μg / ml) at 37°C. When A 600 When it reaches about 0.4~0.6, add 1mM isopropylthio-β-D-galactoside (IPTG) (final concentration 1mM), and continue culturing at 37°C for 3-4 hours to induce the expression of the target product. Then centrifuge the cells and medium, and add buffer components to the bacteria containing the target protein, the final concentration reaches 50mM Tris-HCl, pH8. The precipitate (insoluble part) is the crude extract of the fusion protein.
[0061] The crude extract was washed, denatured and refolded, and the obtained refolded protein was purified by ion exchange chromatography: a DEAE-Sepharose FastFlow column (Pharmacia) equilibrate...
Embodiment 3
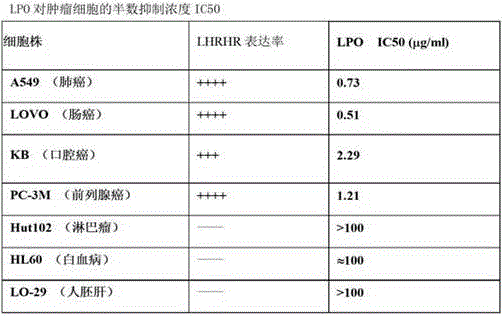
[0062] Example 3: Determination of the biological activity of the fusion protein using an in vitro cultured tumor cell inhibition method (for specific operations, refer to Appendix XC of the third part of the Chinese Pharmacopoeia 2010 edition).
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com