Preparation method of tin of lithium ion battery and conductive polymer composite cathode material membrane
A conductive polymer and lithium-ion battery technology, applied in battery electrodes, lithium batteries, non-aqueous electrolyte batteries, etc., can solve the problems of cumbersome process and difficult large-scale production, and achieve the effect of firm combination and low density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
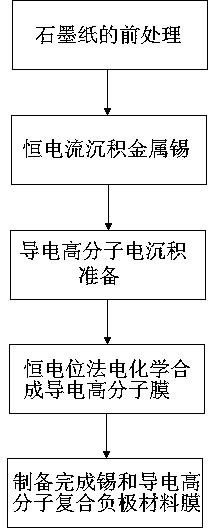
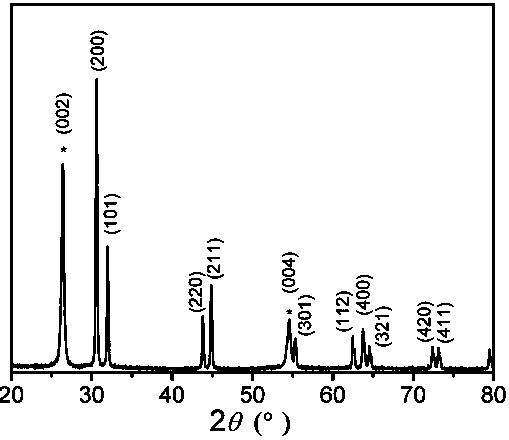
[0031] A preparation method of a tin and conductive polymer composite negative electrode material film for a lithium ion battery, the tin and conductive polymer composite negative electrode material film is based on graphite paper, and the metal tin deposited on the surface of the graphite paper is granular , the size of the metal tin is 0.5-2um, the conductive polymer nanofibers are dispersed among the metal tin particles and on the surface, the diameter of the conductive polymer nanofibers is 0.1-0.8 um, specifically including the following steps:
[0032] a. Pretreatment of graphite paper: the graphite paper is cleaned and surface treated by cleaning liquid in the cleaning tank;
[0033] b. Constant current deposition of metal tin: connect the graphite paper to the external power supply through the electrode brush contact, use the graphite plate as the counter electrode, and place it in the tin electrodeposition tank. The temperature of the tin electrodeposition solution in ...
Embodiment 1
[0044] Graphite paper with a thickness of 0.08mm is cleaned and treated on the surface, and enters the electrodeposition tank of metal tin. Graphite paper is the working electrode (connected to the external power supply through the electrode brush), the graphite plate with a thickness of 1 cm is the counter electrode, and the tin electrodeposition solution configuration: 0.6 mol / L sodium stannate, 0.2 mol / L K 4 P 2 o 7 ·3H 2 O, 0.2 mol / L NaOH, 0.25 mol / L ascorbic acid, 0.05 mol / L H 2 o 2 ; The bath pH=9; The bath temperature is 60 degrees; The electrodeposition current density is 0.6 mA / cm 2 , the deposition time is 5 minutes, and the stirring rate of the bath solution is 180 rpm;
[0045] Put the tin / graphite paper material in the polymer monomer solution tank, stir at the bottom of the tank, and the stirring rate is 120 rpm. Then enter the conductive polymer deposition tank, the tin / graphite paper is the working electrode (connected to the external power supply thro...
Embodiment 2
[0048] Graphite paper with a thickness of 0.2mm is cleaned on the surface and enters the electrodeposition tank of metal tin. Graphite paper is the working electrode (connected to an external power source through electrode brush contact), and a graphite plate with a thickness of 1 cm is the counter electrode. Tin electrodeposition solution configuration: 0.4mol / L sodium stannate, 0.2mol / L K 4 P 2 o 7 ·3H 2 O, 0.2mol / L NaOH, 0.25mol / L ascorbic acid, 0.05mol / L H 2 o 2 ;The bath pH=9;The bath temperature is 60 degrees;The electrodeposition current density is 0.8mA / cm 2 , the deposition time is 10 minutes, and the stirring rate of the bath solution is 180rpm;
[0049] Put the tin / graphite paper material in the polymer monomer solution tank, stir at the bottom, and the stirring speed is 120rpm. Then enter the conductive polymer deposition tank, the tin / graphite paper is the working electrode (connected to the external power supply through the electrode brush), and the counte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com