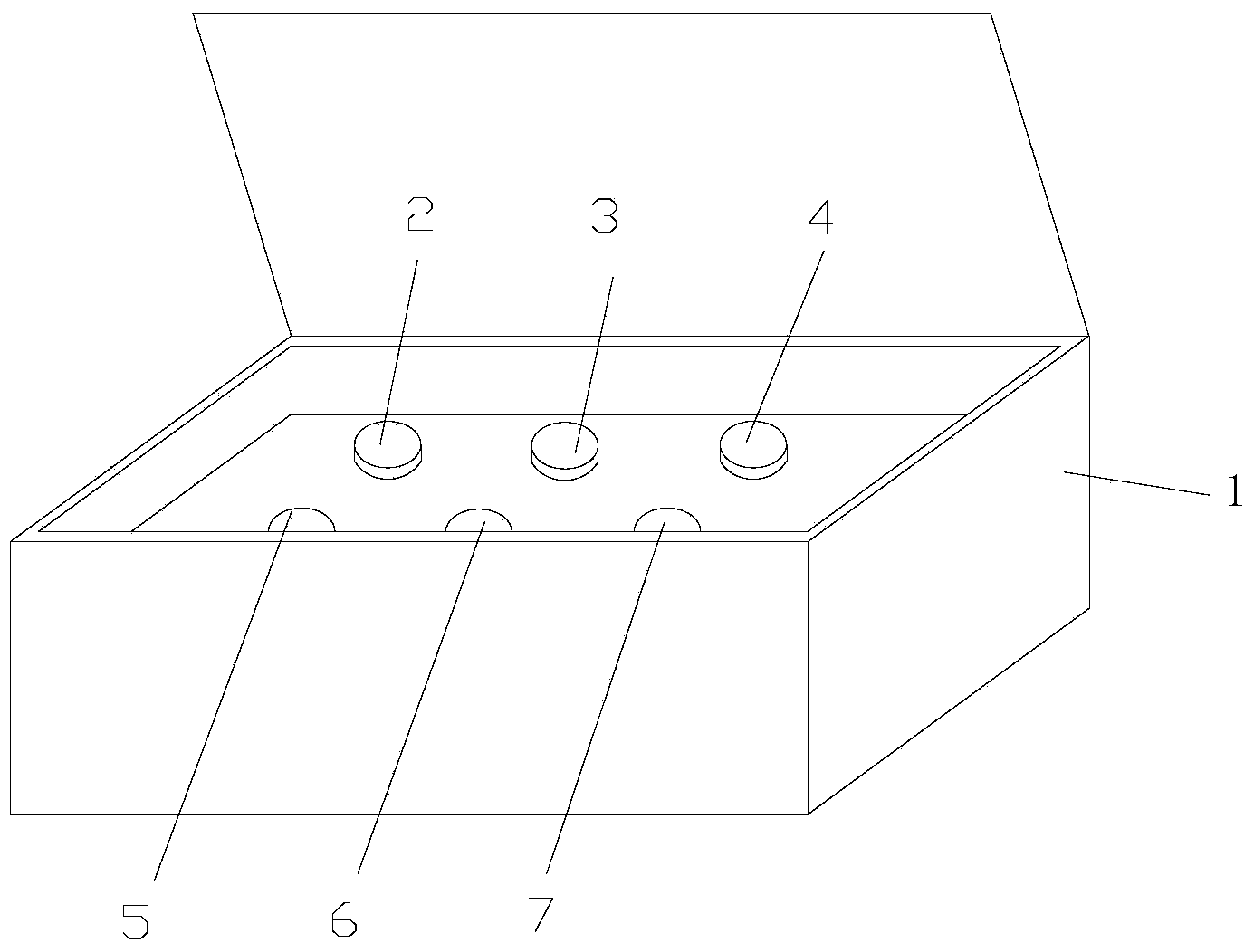
Kit and method for determining mutation sites of genes of acetaldehyde dehydrogenase 2 and methylene tetrahydrofolic acid reductase by virtue of single tube at the same time
A technology of methylenetetrahydrofolate and acetaldehyde dehydrogenase, which is applied in the field of molecular biology testing and can solve the problems of increasing the cost and price of pyrosequencing testing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The key primer components and sequences of the kit are as follows:
[0068] PCR primer 1 (Seq NO1):
[0069] ALDH2Glu504Lys (rs671) upstream primer 5'-CAGTCACCCTTTGGTGGCTACA-3';
[0070] PCR primer 2 (Seq NO2):
[0071] ALDH2Glu504Lys (rs671) downstream primer 5'-CCAGCAGGTCCCACACTCAC-3';
[0072] PCR primer 3 (Seq NO3):
[0073] MTHFR C677T (rs1801133) upstream primer 5'-GGCTGACCTGAAGCACTTGAA-3';
[0074] PCR primer 4 (Seq NO4):
[0075] MTHFR C677T (rs1801133) downstream primer 5'-CAAGTGATGCCCATGTCGGT-3'.
[0076] Sequencing Primer 1 (Seq NO5):
[0077] ALDH2Glu504Lys (rs671) sequencing primer 5'-CACACTCACAGTTTTCACTT-3';
[0078] Sequencing Primer 2 (Seq NO6):
[0079] MTHFR C677T (rs1801133) sequencing primer 5'-TGCGTGATGATGAAAT-3'.
[0080] Take 2 mL of peripheral venous blood of a healthy person A who wants to know about his ability to metabolize ethanol and folic acid, and apply the kit and method of the present invention to carry out acetaldehyde dehydrog...
Embodiment 2
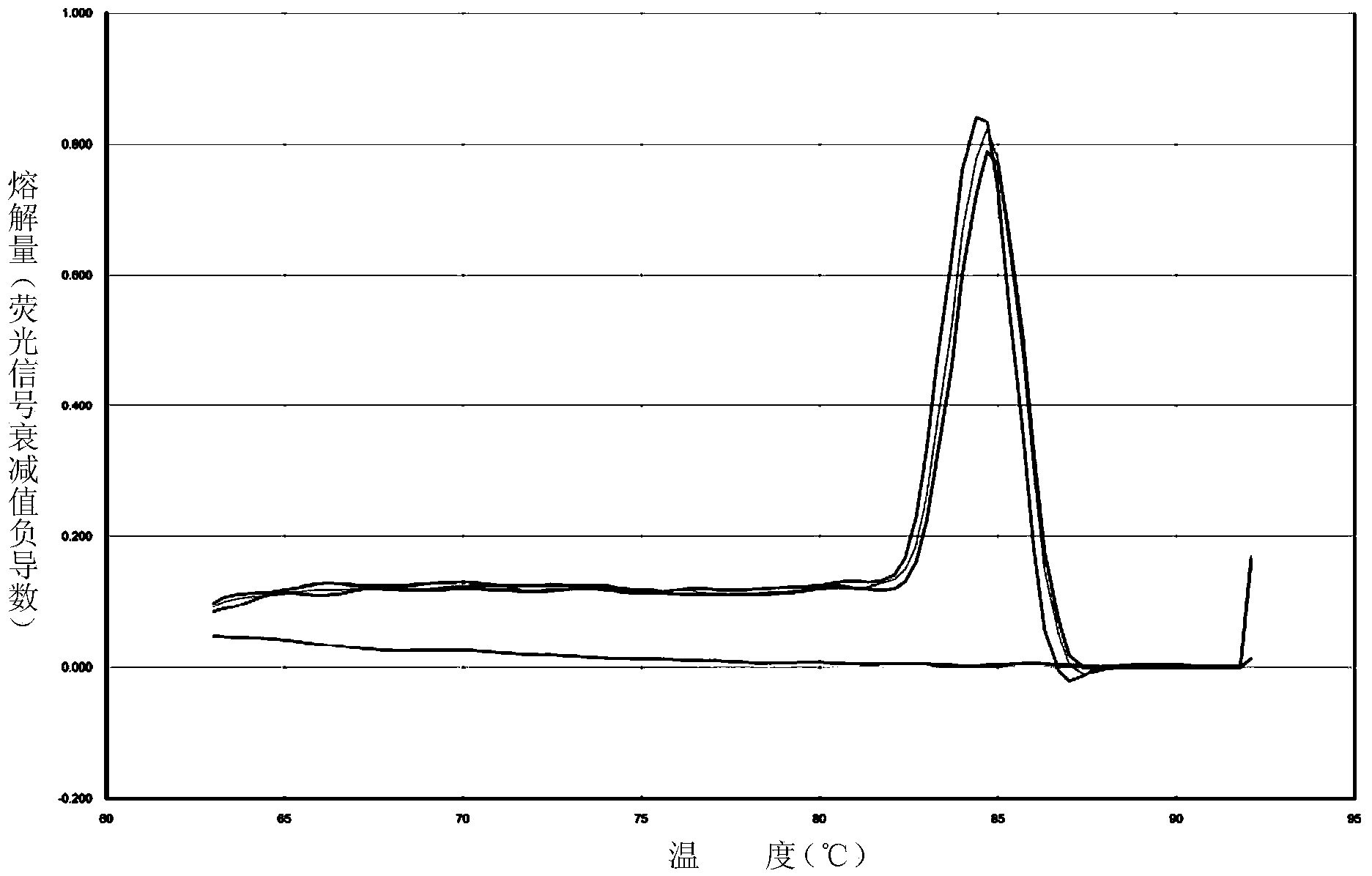
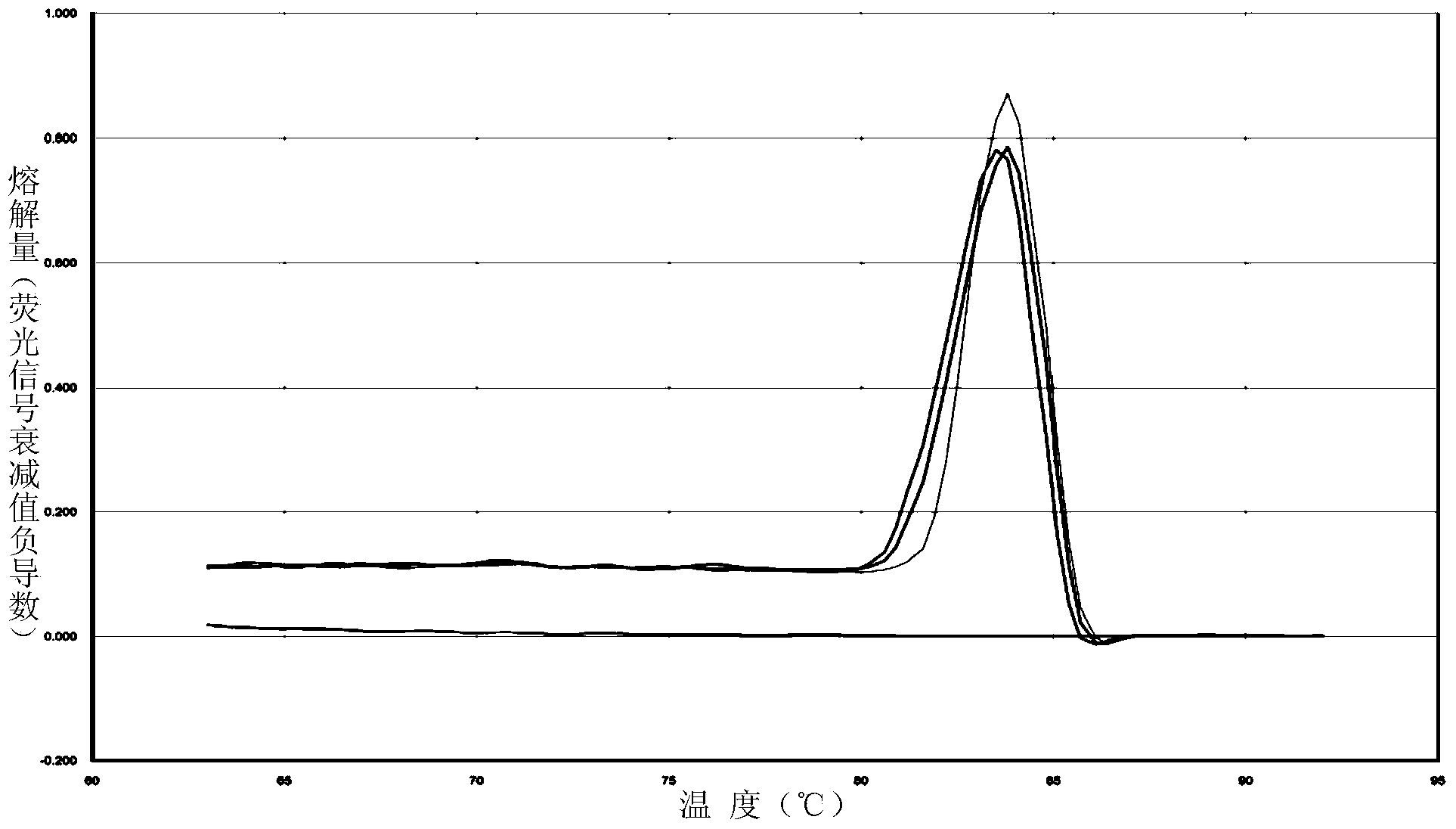
[0200] Pregnant women B and C want to know their own ability to metabolize folic acid to evaluate the impact on fetal development, and pregnant woman C also wants to know the situation of their own ethanol metabolism ability, and the test kit and method of the present invention are used for detection and evaluation. 1 is exactly the same. The MTHFR (rs1801133) test results of pregnant woman B were all the same Figure 7, is G / G genotype; the MTHFR (rs1801133) test results of pregnant woman C are all as Figure 8 , is A / A genotype, the ALDH2 (rs671) test result of pregnant woman C is as follows Figure 5 It is G / G genotype; therefore, pregnant woman B has normal folic acid metabolism capacity, and a normal amount of folic acid supplementation is enough. Pregnant woman B has normal ethanol metabolism capacity, and the risk of diseases related to abnormal ALDH2 genotype is low, but her folic acid metabolism capacity is poor. Need to add more folic acid to prevent adverse effect...
Embodiment 3
[0202] Patient D with a history of myocardial infarction wants to know whether he is suitable to choose nitroglycerin as a quick-acting vasodilator emergency drug, and uses the kit and method of the present invention for detection and evaluation. The detection process is exactly the same as that of Example 1. Results The ALDH2 (rs671) test results of patient D were as follows: Figure 5 It is G / G genotype, the patient has normal metabolism ability to nitroglycerin, and nitroglycerin should be actively and effectively used for vasodilator first aid in this patient.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com