Candida parapsilosis ZJPH1305 and application thereof in preparation of chiral alcohol
A Candida, smoothing technology, applied to Candida parapsilosis and application fields, can solve the problems of easy oxidation and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] 1) Slant culture: Pick a single colony of Candida parapsilosis ZJPH1305 and inoculate it into the slant medium, culture it at 30°C for 2-3 days, and store the slant cells at 4°C in a refrigerator. The final concentration of the slant medium consists of: glucose 15g / L, peptone 7.5g / L, yeast extract 6g / L, (NH 4 ) 2 SO 4 3g / L, KH 2 PO 4 1.5g / L, NaCl0.75g / L, MgSO 4 ·7H 2 O0.75g / L, agar 20g / L, solvent is water, pH6.5.
[0062] 2) Seed culture: Pick a ring of bacteria from the mature slant and insert it into a 250mL shake flask filled with 100mL seed medium, and cultivate at 30°C and 200rpm for 24 hours to obtain a seed liquid. The final concentration of the seed medium consists of: glucose 15g / L, peptone 20g / L, yeast extract 10g / L, (NH 4 ) 2 SO 4 2g / L, KH 2 PO 4 2g / L, NaCl1.0g / L, MgSO 4 ·7H 2 O0.5g / L, the solvent is water, pH6.5.
[0063] 3) Fermentation culture: Transfer the seed solution to a 250mL shake flask containing 100mL fermentation medium with an inoc...
Embodiment 2
[0065] 1) Conversion reaction
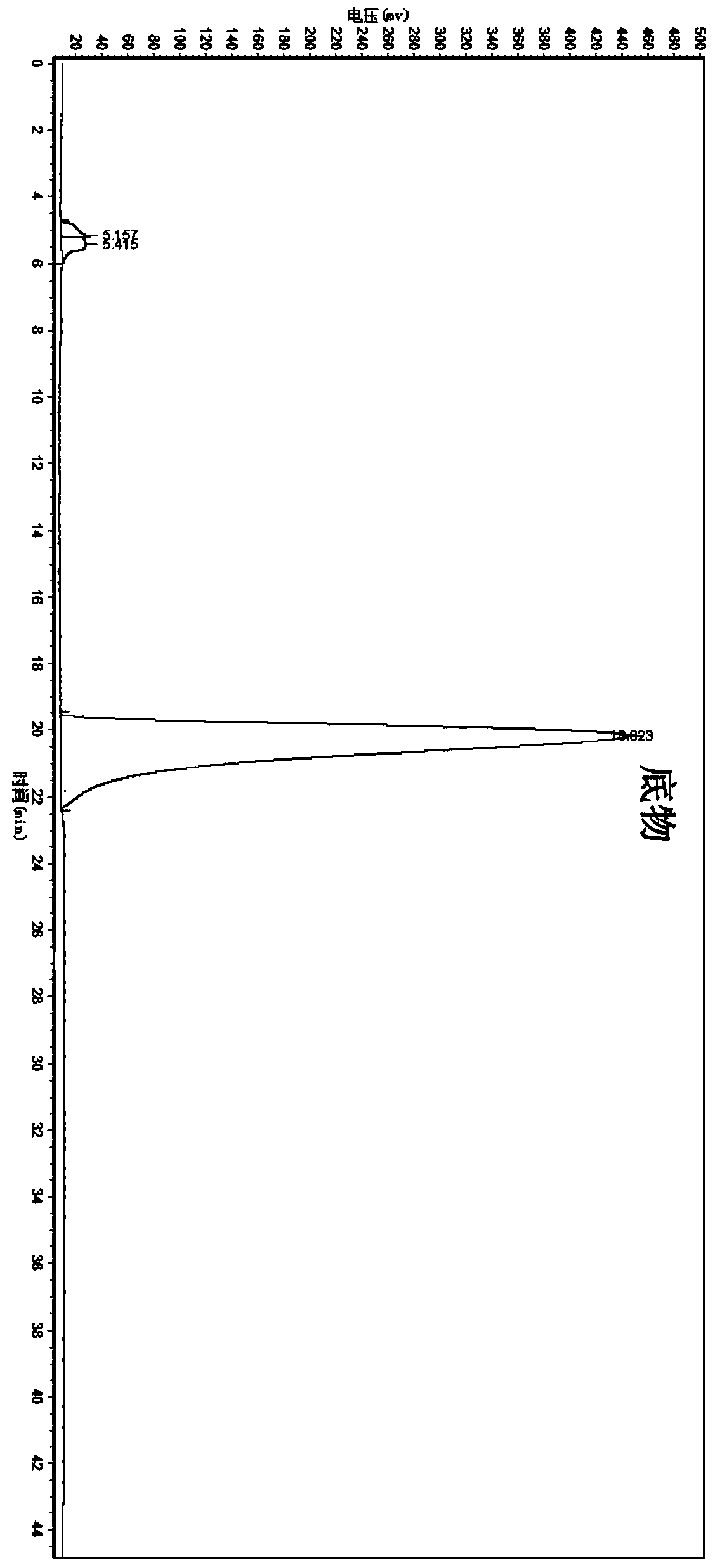
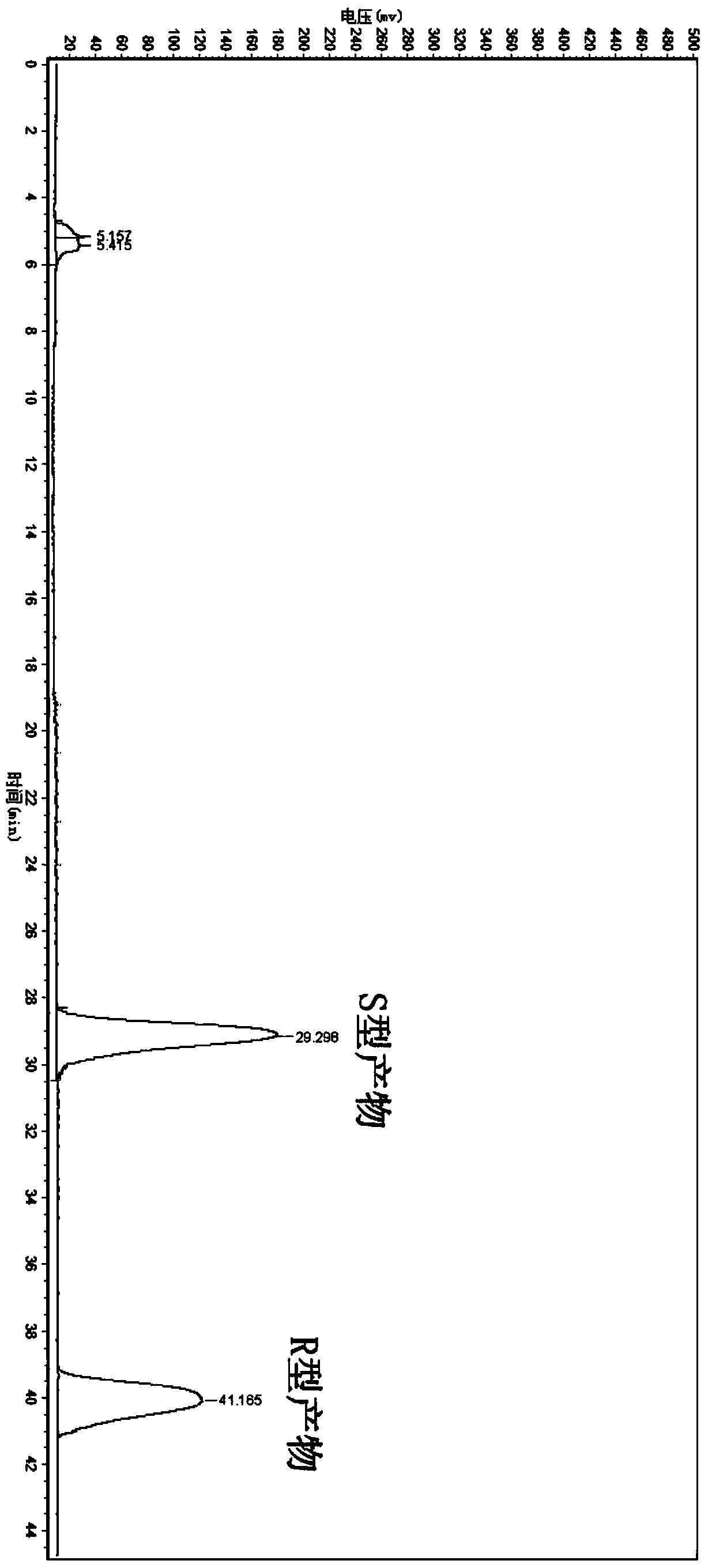
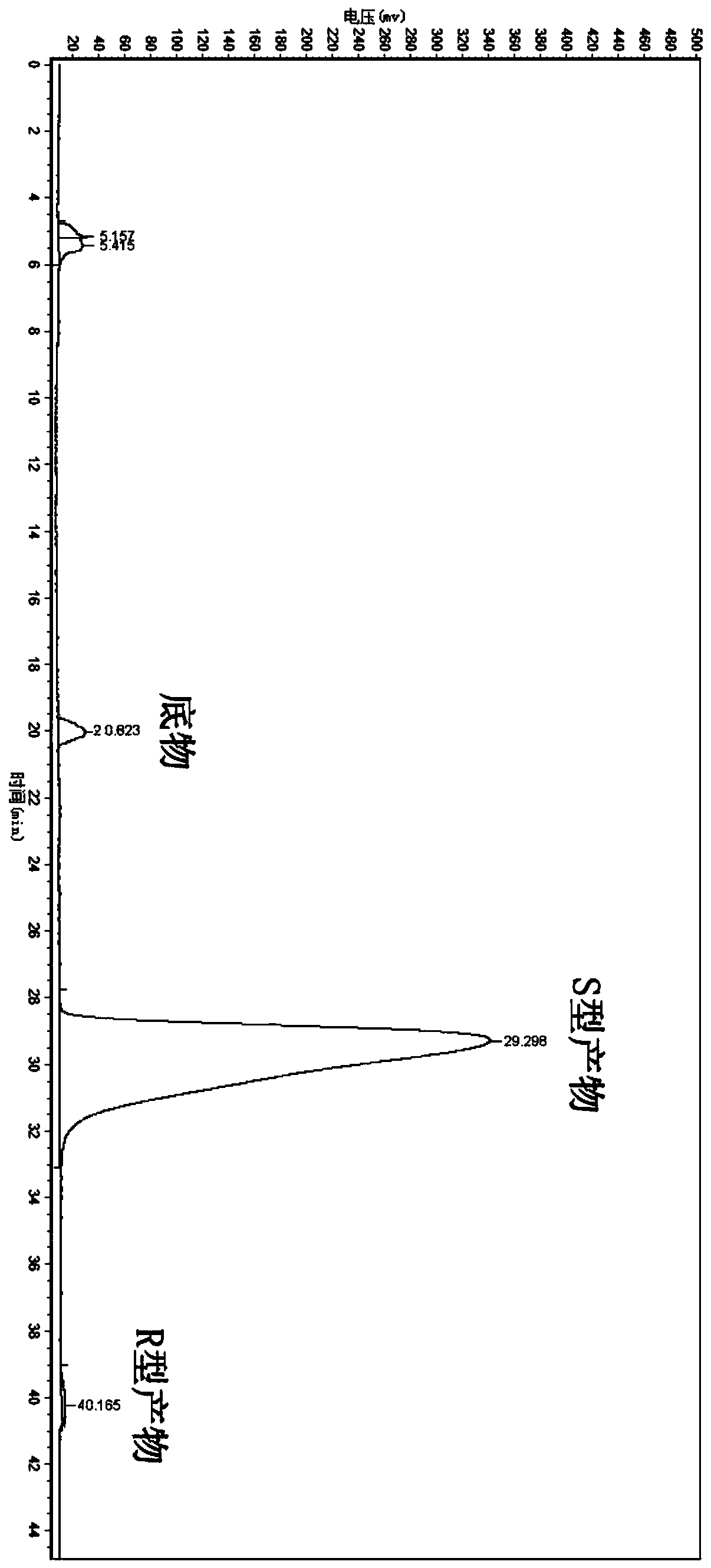
[0066] Suspend the wet bacteria obtained in Example 1 in 10 mL of phosphate buffer (0.2 M, pH 7.0), and the concentration of the wet bacteria is 30.4 g / L based on the dry weight of the bacteria; add the final concentration of 4.76 mmol / L 5-(4-fluorophenyl)-5-ketopentanoic acid was used as a substrate, and sucrose at a final concentration of 10 g / L was added as an auxiliary substrate, and placed in a shaker at 30° C. at 200 rpm for 24 hours. After conversion, the conversion solution was extracted twice with an equal volume of ethyl acetate, and the extract phases were combined to obtain an ethyl acetate solution containing (5S)-(4-fluorobenzene)-5-hydroxyvaleric acid, which was analyzed by liquid chromatography. The content of target product and residual substrate, and the optical purity of the product.
[0067] 5) Analysis and detection:
[0068] The concentrations of products and residual substrates in the conversion reaction extract were ana...
Embodiment 3
[0070] The wet bacteria obtained in Example 1 were suspended in 10 mL of phosphate buffer (0.2M, pH 7.0), and the concentration of the wet bacteria was 30.4 g / L in terms of dry weight of the bacteria; 5 -(4-fluorobenzene)-5-ketopentanoic acid is used as a substrate without an auxiliary substrate (control), placed at 30° C., and reacted in a shaker at 200 rpm for 24 hours. Using the detection method of Example 2, the product ( The concentration of 5S)-(4-fluorobenzene)-5-hydroxyvaleric acid was 0.89mmol / L, the optical purity ee value was 99.9%, and the yield was 18.70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com