The preparation method of asenapine maleate
A technology of asenapine maleate and organic acid, applied in the field of preparation of high-purity asenapine maleate, can solve problems such as low purity of finished product, complicated process, etc., and achieves improved reaction yield, simple operation, Good practical value and the effect of social and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
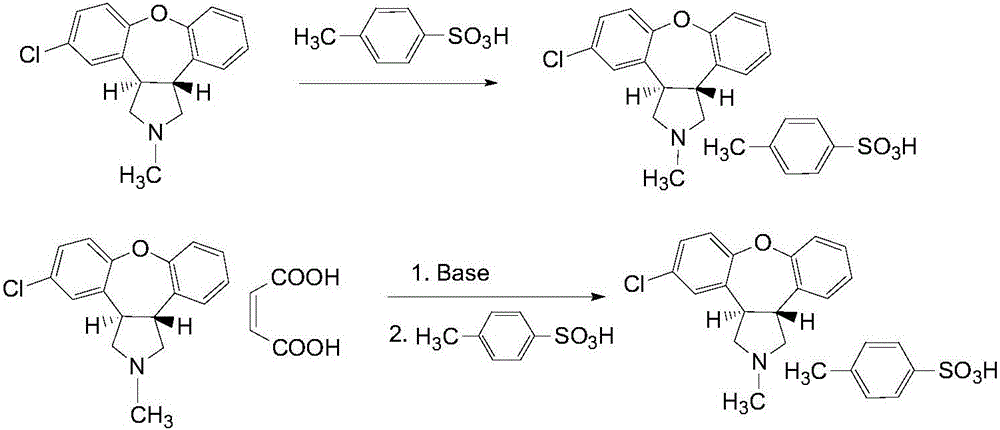
[0021] Example 1 Preparation of high-purity asenapine maleate from crude asenapine free base as raw material
[0022] (1) 1000mL of absolute ethanol is added to the crude product containing 318 grams of asenapine free base (C 17 h 16 ClNO, 1mol, HPLC purity is about 90%) in the pear-shaped flask of jelly, after warming (40-50 ℃) dissolves, then add 190 grams of p-toluenesulfonic acid (C 7 h 8 SO 3 , 1.1mol), after the reaction system is clarified, stop heating and stir for 6-12h, a large amount of white solid precipitates, filter, rinse with a small amount of cold ethanol (ethanol at a temperature of 0-10°C), and vacuum dry at 50°C for 8 hours, Obtain 437 grams of asenapine p-toluenesulfonate, yield 95.4%;
[0023] (2) Add 437 grams of the above-mentioned asenapine p-toluenesulfonate to 1 L of ethyl acetate, slowly add 1 L of 10% (g / mL) sodium hydroxide under stirring, continue stirring for 0.5 hours, and the solution becomes clear; Separate the organic phase, extract the...
Embodiment 2
[0026] Example 2 Preparation of high-purity asenapine maleate from crude asenapine free base as raw material
[0027] (1) 1000mL of absolute ethanol is added to the crude product containing 301 grams of asenapine free base (C 17 h 16 ClNO, 1mol, HPLC purity is about 95%) in the pear-shaped flask of jelly, after warming (40-50 ℃) dissolves, then add 224 grams of p-toluenesulfonic acid (C 7 h 8 SO 3 , 1.3mol), after the reaction system was clarified, stop heating and stir for 6-12h, a large amount of white solid precipitated, filtered, rinsed with a small amount of cold ethanol, and dried in vacuum at 50°C for 8 hours to obtain asenapine p-toluenesulfonate 440 grams, yield 96.1%;
[0028] (2) Add 440 grams of the above-mentioned asenapine p-toluenesulfonate to 1 L of ethyl acetate, slowly add 1 L of 10% (g / mL) sodium hydroxide under stirring, continue stirring for 0.5 hours, and the solution becomes clear; Separate the organic phase, extract the aqueous phase twice with eth...
Embodiment 3
[0030] Example 3 Purification and preparation of high-purity asenapine maleate from crude asenapine maleate as raw material
[0031] 447 grams (1mol, HPLC purity is about 90%) of unqualified asenapine maleate crude product that needs to be reworked in production, join in ethyl acetate (EA), with 10% (g / mL) NaOH, adjust The base pH is 10, extracted with ethyl acetate, the combined organic phases are washed with water, dried with anhydrous MgSO4, and the solvent is evaporated to dryness to obtain 260 grams of asenapine oil; then according to the method in Example 1, first use p-toluenesulfonic acid (TsOH) salification, crystallization and purification, then use alkali dissociation and then salify with maleic acid, obtain 346 grams of asenapine maleate after drying, the total yield is 86.1%, and the purity is greater than 99% (HPLC area is normalized chemical method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com