Medicine composition consisting of methylprednisolone aceponate and zinc oxide
A kind of technology of methylprednisolone acetonide and composition, applied in the pharmaceutical composition that zinc oxide is an active ingredient, in the field of methylprednisolone acetonide, the problems such as the change of preparation pH value can be solved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
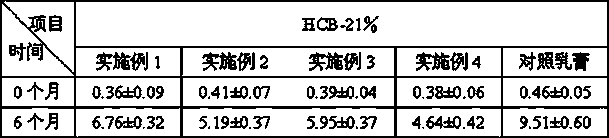
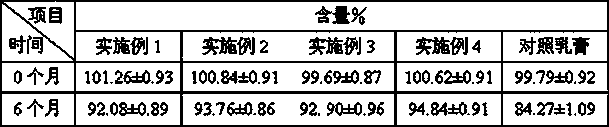
[0022] Active Ingredients: Methylprednisolone Acetate 1g, Zinc Oxide 10g
[0023] Oil phase ingredients: white petrolatum 50g, stearyl alcohol 30g, liquid paraffin 30g, surfactant 1
[0024] Water phase ingredients: surfactant 2, glycerin 50g, propylene glycol 20g, ethylparaben 1g, citric acid (C 6 h 8 o 7 ·H 2 O) 10g, sodium citrate (Na 3 C 6 h 5 o 7 2H 2 O) Add 18g water for injection to 1000g
[0025] Accurately weighed according to the above ratio, the cream preparation process is as follows:
[0026] (1) Oil phase preparation: mix zinc oxide and oil phase ingredients heated to melt in a container, and keep the temperature at 90°C;
[0027] (2) Water phase preparation: Dissolve surfactant 2, citric acid, sodium citrate, and ethylparaben in water for injection, evenly disperse methylprednisolone acetate in glycerin and propylene glycol, mix the two, and heat , Stir evenly and keep the temperature at 90°C;
[0028] (3) Combine the phases: slowly add the oil phase...
Embodiment 2
[0040] Active Ingredients: Methylprednisolone Acetate 1g, Zinc Oxide 5g
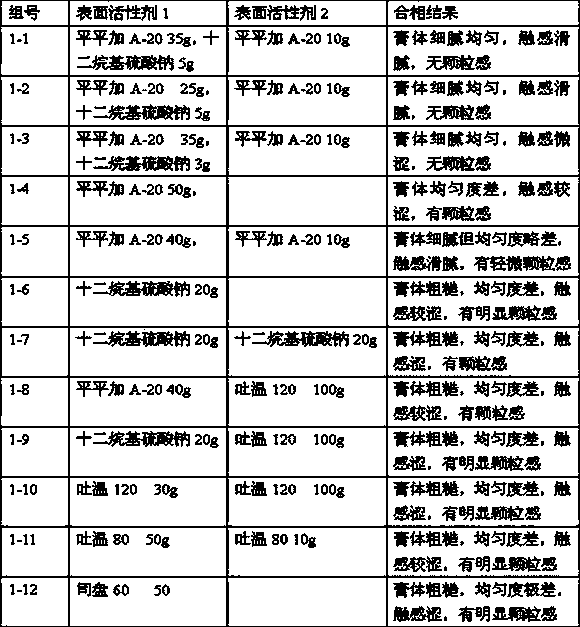
[0041] Oil phase ingredients: white petrolatum 50g, stearyl alcohol 30g, liquid paraffin 30g, Pingpingjia A-20 35g, sodium lauryl sulfate 5g
[0042] Water phase ingredients: Pingpingjia A-20 10g, glycerin 50g, propylene glycol 20g, ethylparaben 1g, citric acid (C 6 h 8 o 7 ·H 2 O) 10g, sodium citrate (Na 3 C 6 h 5 o 7 2H 2 O) Add 18g water for injection to 1000g
Embodiment 3
[0044] Active Ingredients: Methylprednisolone Acetate 0.1g, Zinc Oxide 5g
[0045] Oil phase ingredients: 30g white petrolatum, 100g stearyl alcohol, 30g liquid paraffin, 25g Pingpingjia A-20, 5g sodium lauryl sulfate
[0046] Water phase ingredients: Pingpingjia A-20 20g, glycerin 50g, propylene glycol 50g, methylparaben 1g, citric acid (C 6 h 8 o 7 ·H 2 O) Add 7g water for injection to 1000g
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com