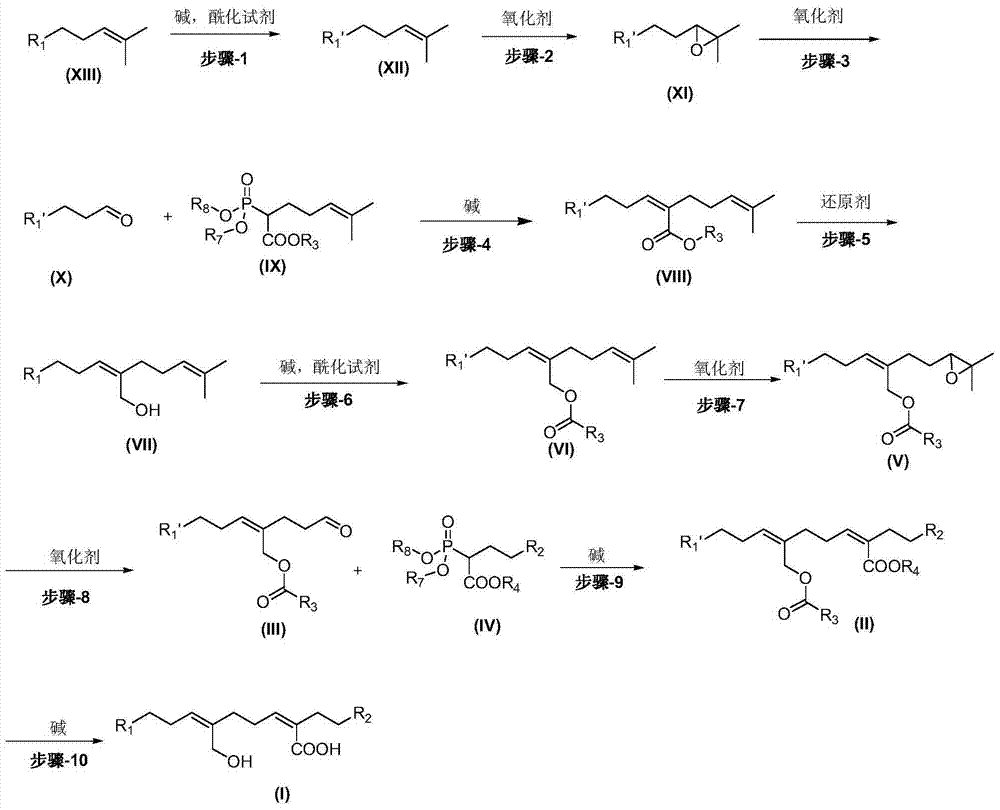
Preparation method of smallanthus sonchifolitus diterpene acid compound and compound obtained by using preparation method
A technology of agonist diterpene acids and compounds, applied in the field of chemical synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
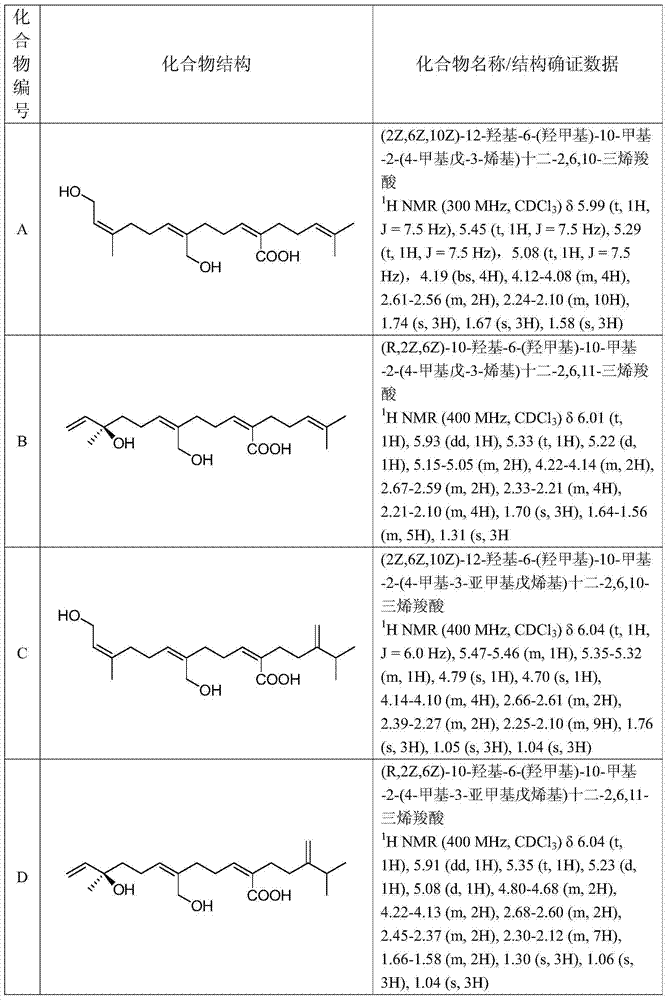
[0106] Example 1: (2Z,6Z,10Z)-12-Hydroxy-6-(hydroxymethyl)-10-methyl-2-(4-methylpent-3-enyl)dodeca - Preparation of 2,6,10-trienecarboxylic acid (Compound A)
[0107] The synthetic route of compound A is as follows:
[0108]
[0109]
[0110] Synthesis of Compound IX-a
[0111]
[0112] Add methylmagnesium iodide (166mL, 500mmol) into 400mL of anhydrous ether, and cool to 0°C in an ice-salt bath. To this solution was slowly added dropwise a solution of cyclopropylmethyl ketone (42 g, 500 mmol) in anhydrous ether (100 mL). During the dropwise addition, the temperature should not be higher than 5°C. After the dropwise addition was completed, stirring was continued at room temperature for 2 hours. Slowly pour this reaction solution into a solution of dilute sulfuric acid (200mL concentrated sulfuric acid, 500mL water) cooled to 0°C in advance. Stir at room temperature for an additional 1 hour. The aqueous phase obtained by liquid separation was extracted with eth...
Embodiment 2
[0147] Example 2: (2Z,6Z,10Z)-12-Hydroxy-6-(hydroxymethyl)-10-methyl-2-(4-methyl-3-methylenepentenyl)dodeca-2 , Synthesis of 6,10-trienecarboxylic acid (Compound C)
[0148] The synthetic route of compound C is as follows:
[0149]
[0150] Synthesis of Compound IV-b
[0151]
[0152] 2,3-Dimethyl-1-butene (8.3g, 98.16mmol) and paraformaldehyde (2.46g, 81.8mmol) were successively added to 170mL of anhydrous dichloromethane, cooled to 0°C in an ice bath. To this solution was slowly added dropwise Me 2AlCl in n-hexane (100 mL, 90 mmol, 0.9 M). After the dropwise addition was completed, stirring was continued overnight at room temperature. Pour 5mL NaH into the reaction solution under ice bath 2 PO 4 solution, precipitated, and 10% hydrochloric acid was added dropwise until the precipitate was completely dissolved. The organic phase was separated, the aqueous phase was extracted with dichloromethane (2x200 mL), the combined organic phases were dried over anhydrous so...
Embodiment 3
[0161] Example 3: (R,2Z,6Z)-10-Hydroxy-6-(hydroxymethyl)-10-methyl-2-(4-methylpent-3-enyl)dodeca-2,6, Synthesis of 11-trienecarboxylic acid (compound B)
[0162] The synthetic route of compound B is as follows:
[0163]
[0164] Synthesis of Compound XII-b
[0165]
[0166] 4-Dimethylaminopyridine (50 g, 410 mmol) and acetic anhydride (80 mL) were sequentially added to a solution of (-)-linalool (46 g, 300 mmol) in 800 mL of acetonitrile. The reaction solution was heated to reflux, and continued to reflux overnight with stirring. After the reaction solution was cooled, 1.5 L of ethyl acetate was added for dilution, washed with 0.1N HCl solution (1 L) and saturated brine (1 L) successively, and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and purified by column chromatography (200-300 mesh normal phase column chromatography silica gel, mobile phase petroleum ether: ethyl acetate = 10:1) to obtain XII-b, 44g, a colorless oil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com