Wholly aromatic side-chain-type sulfonated dichloro monomer and preparation method thereof
A technology for sulfonating dichloride and dichloride monomers, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as the limitation of sulfonic acid group activity, and achieve the effect of improving proton conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
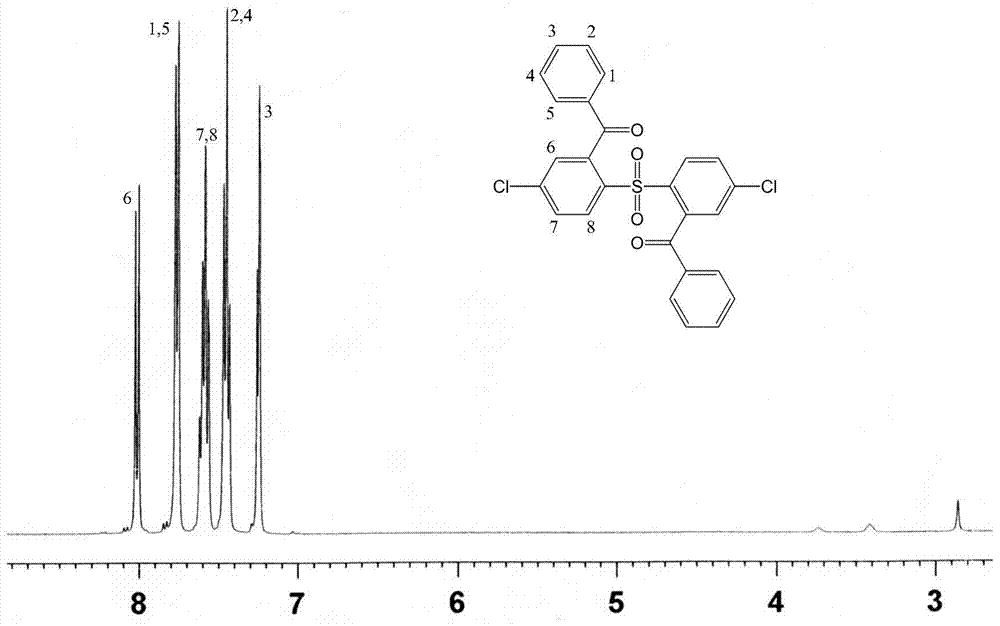
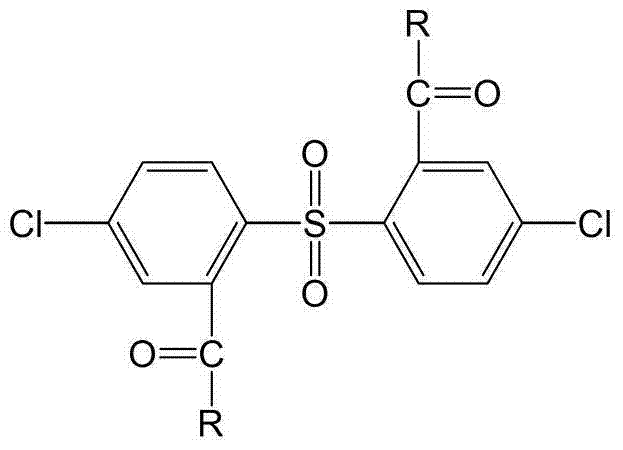
[0028] A fully aromatic side chain type sulfonated dichloro monomer, the structural formula and reaction formula are as follows:
[0029]
[0030] Preparation of 2,2'-benzophenone-4,4'-dichlorodiphenylsulfone:
[0031] Under the condition of nitrogen protection and magnetic stirring, 11.5g (40mmol) of 4,4'-dichlorodiphenyl sulfone and 200mL tetrahydrofuran solvent were added to a dry 500mL reaction flask, and the temperature of the system was lowered to Below -60°C, add 33mL (84mmol) of n-butyllithium solution dropwise to the reaction system, and keep the temperature not higher than -55°C, react for 2 hours after the dropwise addition, then add 11.45g (84mmol) of dry zinc chloride, React at low temperature below -60°C for 2 hours, add 16.0g (84mmol) of cuprous iodide, gradually increase the temperature from -60°C to -30°C for 1 hour, finally add 11.8g (84mmol) of benzoyl chloride, and control the temperature at React below -10°C for more than 5 hours. After the reaction, w...
Embodiment 2
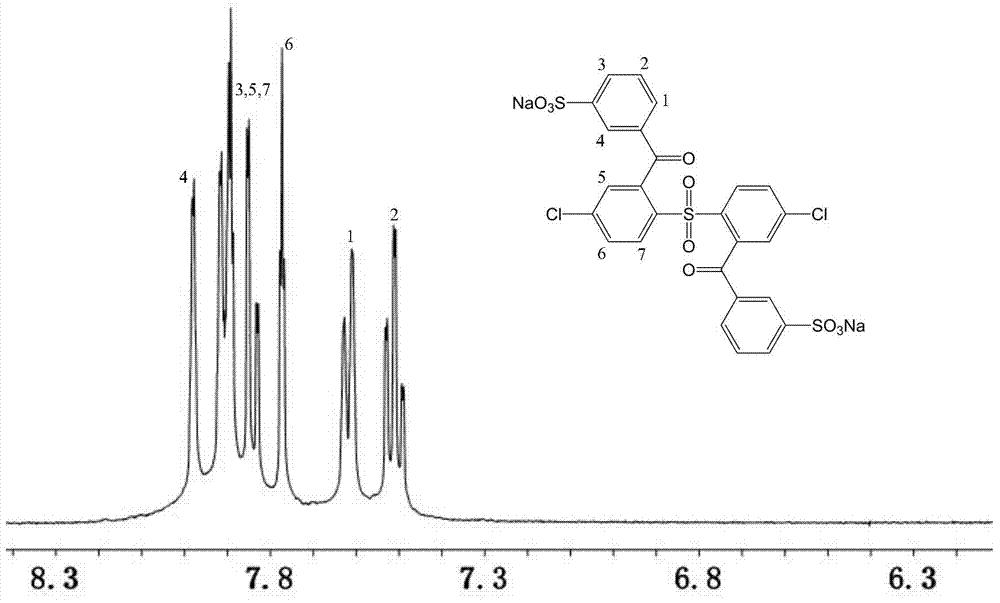
[0039] A fully aromatic side chain type sulfonated dichloro monomer, the structural formula and reaction formula are as follows:
[0040]
[0041] Preparation of 2,2'-benzophenone-4,4'-dichlorodiphenylsulfone:
[0042]Under the condition of nitrogen protection and magnetic stirring, 11.5g (40mmol) of 4,4'-dichlorodiphenyl sulfone and 200mL tetrahydrofuran solvent were added to a dry 500mL reaction flask, and the temperature of the system was lowered to Below -60°C, add 33mL (84mmol) of n-butyllithium solution dropwise to the reaction system, and keep the temperature not higher than -55°C, react for 2 hours after the dropwise addition, then add 11.45g (84mmol) of dry zinc chloride, React at low temperature below -60°C for 2 hours, add 7.52g (84mmol) of cuprous nitrile, and gradually raise the temperature from -60°C to -30°C for 1 hour, finally add 11.8g (84mmol) of benzoyl chloride, and control the temperature at React below -10°C for more than 5 hours. After the reaction, ...
Embodiment 3
[0046] A fully aromatic side chain type sulfonated dichloro monomer, the structural formula and reaction formula are as follows:
[0047]
[0048] Preparation of 2,2'-di-p-phenoxybenzophenone-4,4'-dichlorodiphenylsulfone:
[0049] Under nitrogen protection and magnetic stirring conditions, add 11.5g (40mmol) of 4,4'-dichlorodiphenyl sulfone and 240mL tetrahydrofuran solvent to a dry 500mL reaction flask, and use a liquid nitrogen-acetone low temperature bath to reduce the system temperature to Below -60°C, add 33mL (84mmol) of n-butyllithium solution dropwise to the reaction system, and keep the temperature not higher than -55°C, react for 2 hours after the dropwise addition, then add 11.45g (84mmol) of dry zinc chloride, React at a low temperature below -60°C for 2 hours, add 16.0g (84mmol) of cuprous iodide, gradually increase the temperature from -60°C to -30°C for 1 hour, and finally add 19.5g (84mmol) of 4-phenoxybenzyl Acyl chloride, the temperature is controlled bel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com