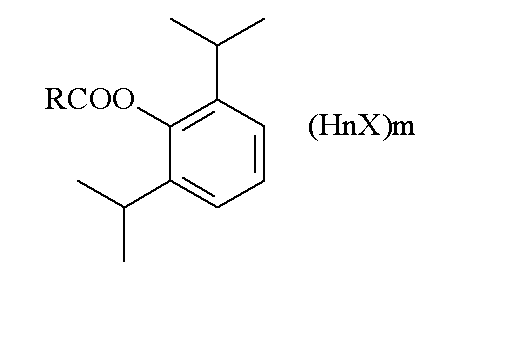
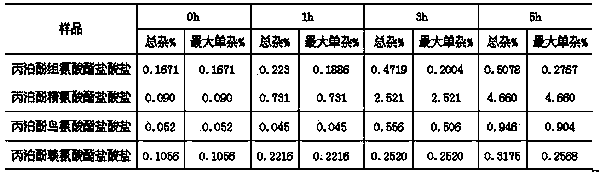
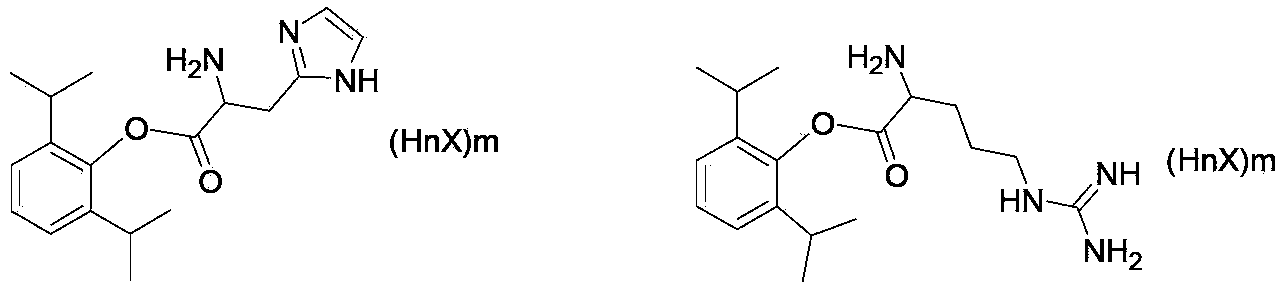
Alkaline amino acid ester salt of propofol
A technology of propofol basicity and amino acid ester, applied in the field of medicine, can solve the problems of difficult to obtain practical application, short compound induction time and duration, and achieve the effects of easy large-scale preparation, good water solubility and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the synthesis of propofol histidine ester hydrochloride
[0047] Dissolve histidine (24g, 0.16mol) in a mixed solvent of 2M NaOH solution (300ml) and 1,4-dioxane (200ml), cool down to 0°C, and add di-tert-dicarbonate dropwise to the above system Butyl ester (176g, 0.805mol) in 1, 4-dioxane (200ml) was added dropwise and stirred overnight at 25°C. Add water (500ml) to the reaction system, stir, and extract the aqueous phase with petroleum ether three times (500ml×3). Add appropriate amount of citric acid (200g) to adjust the water phase to weak acidity (pH=6). The aqueous phase was extracted twice with ethyl acetate (500ml×2), and the organic phases were combined and washed three times with saturated brine (500ml×3). Dry over magnesium sulfate, filter, and wash the filter cake with ethyl acetate. The filtrate was concentrated to obtain N-Boc-protected histidine (Boc-His(Boc)-OH), a white solid, weight: 50 g, yield: 89%.
[0048] Propofol (37.6g, 0.211mo...
Embodiment 2
[0056] Embodiment 2: the synthesis of propofol arginine hydrochloride
[0057] Dissolve arginine (21g, 0.121mol) in a mixed solvent of 2M NaOH solution (300ml) and 1,4-dioxane (100ml), then cool down to 0°C, and add dicarbonate dicarbonate dropwise to the above system The solution of tert-butyl ester (92.5g, 0.424mol) in 1,4-dioxane (100ml) was added dropwise and stirred overnight at 25°C. Add water (500ml) to the reaction system, stir, and extract the aqueous phase with petroleum ether three times (200ml×3). Add appropriate amount of citric acid (200g) to adjust the pH value of the aqueous phase to weak acidity (PH=6). The aqueous phase was extracted twice with ethyl acetate (200ml×2), and the organic phases were combined and washed three times with saturated brine (200ml×3). Dry over magnesium sulfate, filter, and wash the filter cake with ethyl acetate. Concentrate filtrate, obtain the arginine (Boc-Arg(Boc) of N-Boc protection 2 )-OH), white solid, weight: 46g, yield: ...
Embodiment 3
[0066] Embodiment 3: the synthesis of propofol ornithine hydrochloride
[0067] Ornithine hydrochloride (37.6g, 0.183mol) was dissolved in a mixed solvent of 2M NaOH solution (350ml) and 1,4-dioxane (150ml), then cooled to 0°C, and added dropwise to the above system Di-tert-butyl dicarbonate (120g, 0.550mol) in 1,4-dioxane (200ml) was added dropwise and stirred overnight at 25°C. Add water (500ml) to the reaction system, stir, and extract the aqueous phase with petroleum ether three times (500ml×3). Add appropriate amount of citric acid (80g) to adjust the pH value of the aqueous phase to weak acidity (PH=6). The aqueous phase was extracted twice with ethyl acetate (500ml×2), and the organic phases were combined and washed three times with saturated brine (500ml×3). Dry over magnesium sulfate, filter, and wash the filter cake with ethyl acetate. The filtrate was concentrated to obtain N-Boc-protected ornithine (Boc-Orn(Boc)-OH), a white solid, weight: 56 g, yield: 92%. . ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com