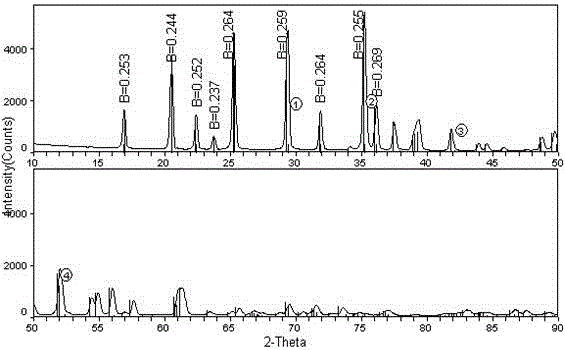
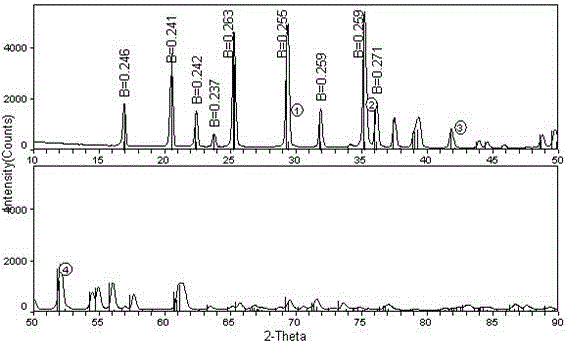
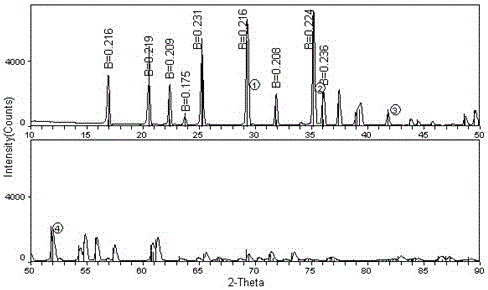
Lithium-ion battery cathode active material limn x fe 1-x po 4 The preparation method of /c
A positive electrode active material, lithium-ion battery technology, applied in the direction of battery electrodes, secondary batteries, chemical instruments and methods, etc., can solve the problem that lithium iron manganese phosphate is not a uniform single-phase crystal, and achieve uniformity consistent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The invention provides a lithium ion battery cathode active material LiMn x Fe 1-x PO 4 The preparation method of / C, the method may further comprise the steps:
[0024] S1, mixing soluble divalent manganese salt and soluble ferrous salt evenly to form a manganese-ferric salt mixture;
[0025] S2. Under the protection of inert gas, mix ferromanganese salt with complex precipitation agent, and prepare Mn by co-precipitation x Fe 1-x C 2 o 4 2H 2 O precursor;
[0026] S3, washing and drying the precursor to obtain precursor powder;
[0027] S4, under the protection of inert gas, the Mn x Fe 1-x C 2 o 4 2H 2 O precursor is mixed with soluble lithium salt and phosphate to obtain a precursor solution;
[0028] S5, reacting the precursor solution in an airtight container protected by an inert gas to obtain a reactant solution;
[0029] S6. After emulsifying and dispersing the carbon source into the reactant solution, dry it to obtain a mixture powder, sinter th...
Embodiment 1
[0053] 1L of 0.1mol / l concentration of ferrous sulfate and 1L of 0.1mol / l concentration of manganous sulfate solution were added dropwise to 1L of 0.2mol / l concentration of oxalic acid solution, while using ammonia to control the pH of the reaction solution to 7. Stir and react under circulating water at 40°C for 3 hours. After the reaction solution is left to age for 24 hours, filter, wash and dry to obtain light yellow Mn 0.5 Fe 0.5 C 2 o 4 2H 2 O Material A1.
[0054] 0.1mol of Mn 0.5 Fe 0.5 C 2 o 4 2H 2 O powder, 0.1mol lithium hydroxide, and 0.1mol phosphoric acid were added to 1L of deionized water, and the mixed solution was ball-milled in a ball mill for 1 hour, and then the solution after ball milling was transferred to a hydrothermal reaction kettle, under the protection of N2 at 180°C, After reacting for 16 hours under 1000rpm stirring, it was naturally cooled to room temperature, and the solution after the reaction was filtered, washed and dried 4 times to...
Embodiment 2
[0057] Add 1L of 0.4mol / l concentration of ferrous nitrate and 1L of 0.8mol / l concentration of manganous nitrate solution to 2L of 0.6mol / l concentration of sodium oxalate solution at the same time, and use sodium hydroxide to control the concentration of the reaction solution. The pH is 7.8, stirred and reacted for 4 hours under circulating water at 60°C, and the reacted solution was left to age for 48 hours, filtered, washed and dried to obtain light yellow Mn 0.67 Fe 0.33 C 2 o 4 2H 2 O material A2.
[0058] 0.5mol of Mn 0.67 Fe 0.33 C 2 o 4 2H 2 O powder and 0.5mol lithium dihydrogen phosphate were added to 1L of deionized water respectively, and the mixed solution was sand-milled in a sand mill for 2 hours, and the sand-milled solution was transferred to a hydrothermal reaction kettle. 1000rpm stirred and reacted for 24 hours, then naturally cooled to room temperature, and the solution after the reaction was filtered, washed and dried 4 times to obtain LiMn 0.67...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com