Igg-binding peptide and method for detecting and purifying igg using same
A residue and amino acid technology, applied in the field of human IgG-binding peptides, can solve problems such as utilization limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0349] Hereinafter, the present invention will be described more specifically with reference to examples, but the scope of the present invention is not limited by these examples.
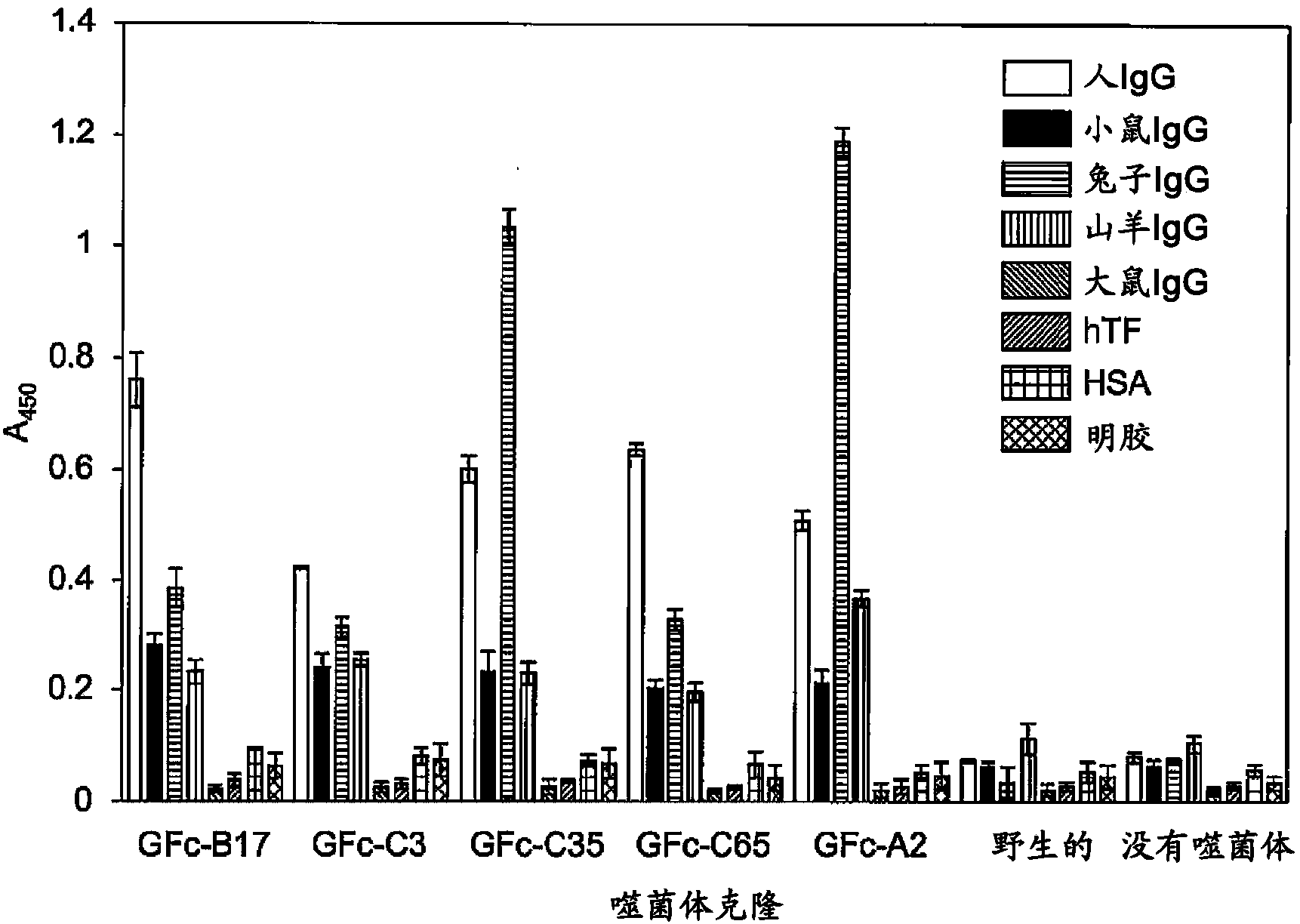
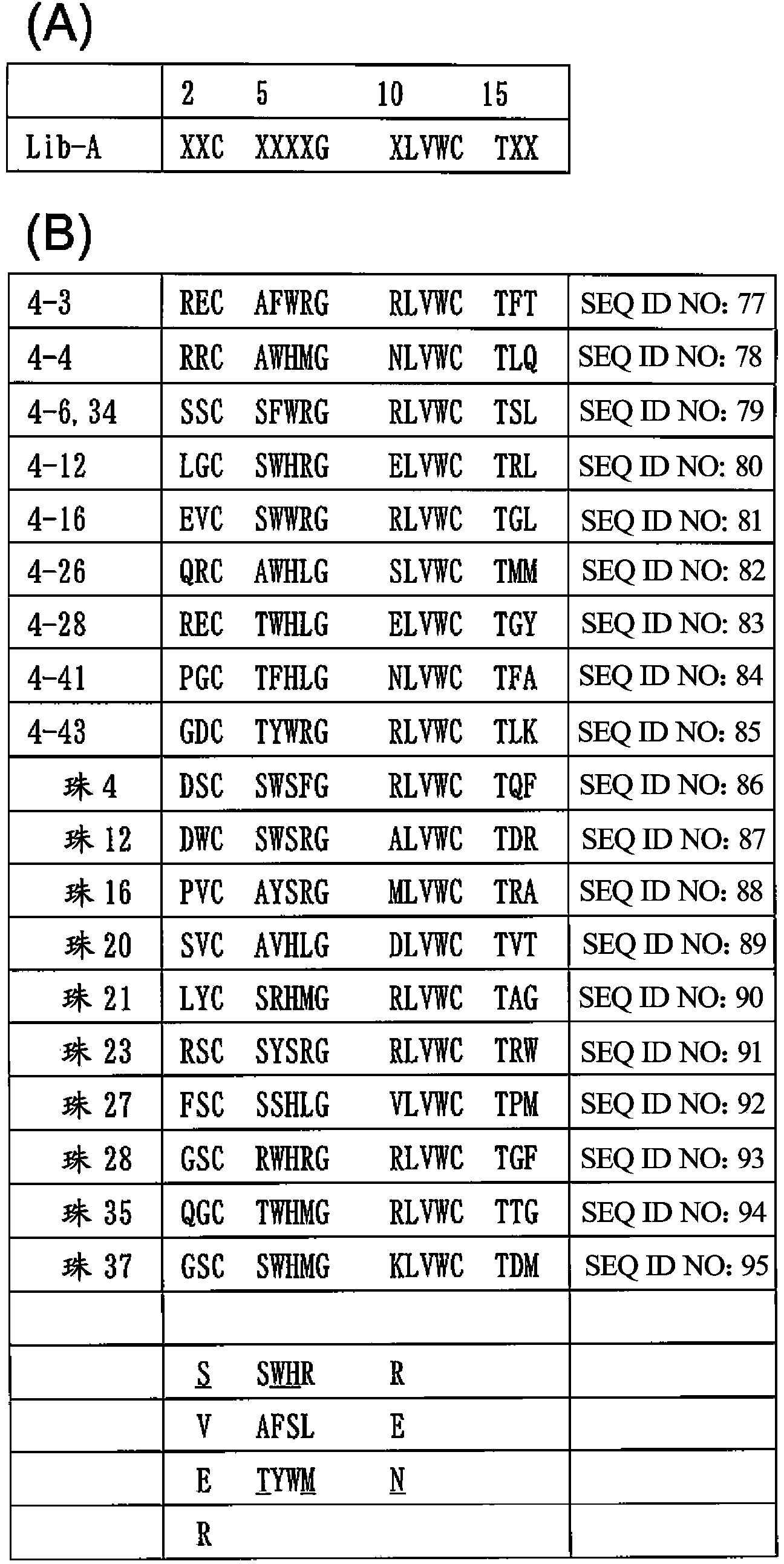
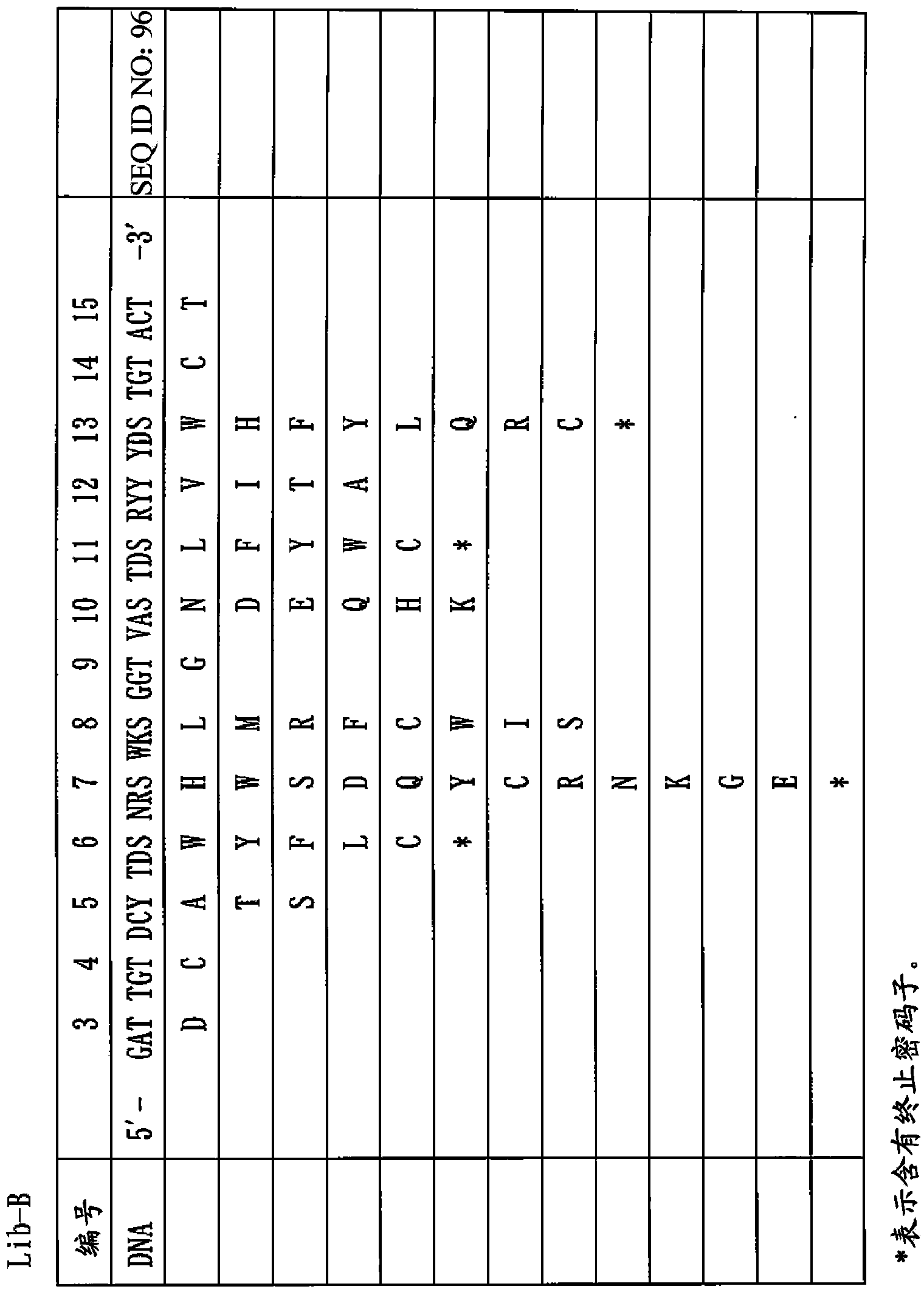
[0350] In order to isolate human IgG-specific phage from a random peptide library having a circular structure formed by two Cys constructed by the T7 phage display method, the following biopanning method was used.
[0351] That is, 5 × 10 10 pfu T7 phage library (X 3 CX 8 CX 3 、X 3 CX 9 CX 3 x 3 CX 10 CX 3 ) solution was added to a 96-well microplate coated with human IgG-Fc (from human plasma, Athens Research & Technology, Athens, GA, USA) (1 μg / 100 μl / well) and blocked with 0.5% BSA (Nunc, Maxisorp ) wells and allowed to react for 1 hour. After removing the supernatant phage solution, the wells were washed 10 times with PBS containing 0.1% Tween. Escherichia coli BLT5615 (Novagen) culture solution (300 μl) was added to infect it, and cultured together with 3 ml of Escherichia coli cultu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com