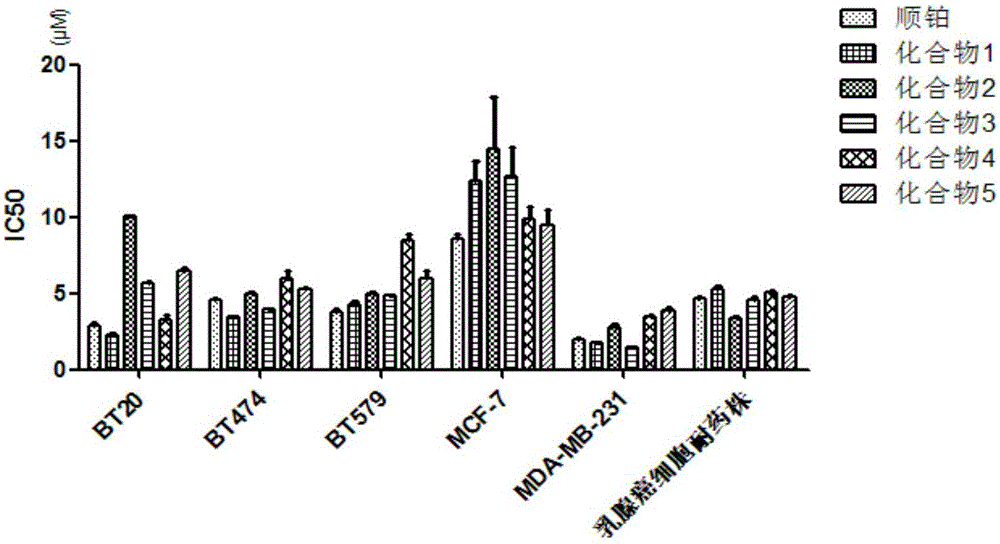
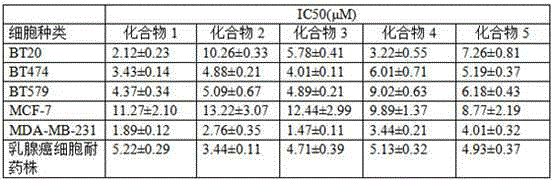
2-(3,4-dimethoxy)benzoyl-5-(4-substituted phenylethynyl)thiophene and its preparation method and application
A benzoyl and dimethoxy technology, applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problem of cardiotoxicity, inappropriate treatment, and inaccurate treatment duration Sexuality and other issues, to achieve the effect of significantly inhibiting proliferation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The preparation method of 2-(3,4-dimethoxy)benzoyl-5-(4-substituted phenylethynyl)thiophene comprises the following steps:
[0031] 1) In the presence of a catalyst, fully react 2-bromothiophene and 3,4-dimethoxybenzoyl chloride in a solvent, add acid after the reaction, remove the solvent, and extract the residue with an organic extractant The extractant layer is obtained, washed with water, and then the extractant layer is dried with a desiccant to remove the organic extractant in the extractant layer, and the residue is separated and purified to obtain 2-(3,4-dimethoxy)benzoyl-5 - Bromothiophene;
[0032] 2) In the presence of tetrakis(triphenylphosphine)palladium, CuI, and acid-binding agent, 2-(3,4-dimethoxy)benzoyl-5-bromothiophene, 4-substituted phenylacetylene Fully react in an organic solvent, remove the solvent after the reaction is completed, and separate and purify the residue to obtain the product.
[0033] In step 1), the molar ratio of 2-bromothiophene ...
Embodiment 1
[0044] The preparation method of described 2-(3,4-dimethoxy)benzoyl-5-(4-substituted phenylethynyl)thiophene comprises the following steps:
[0045] 1) Dissolve 1mmol of 2-bromothiophene in 5ml of dry dichloromethane, add 1.5mmol of anhydrous aluminum trichloride, add dropwise 1mmol of 3,4-dimethoxybenzoyl chloride (dissolved in 3ml of dichloromethane) at 0oC solution. During the dropwise addition, nitrogen protection was applied, and after the addition was completed, the temperature was raised to room temperature and stirred for 6 hours. After the reaction is completed, add 20 wt% dilute hydrochloric acid to decompose aluminum trichloride, remove the solvent under reduced pressure, extract the residue with ethyl acetate, and wash with water. The organic layer was dried with anhydrous sodium sulfate, the solvent was removed under reduced pressure, and the residue was separated and purified by column chromatography to obtain a solid with a yield of 78%;
[0046] 2) Dissolve 1mm...
Embodiment 2
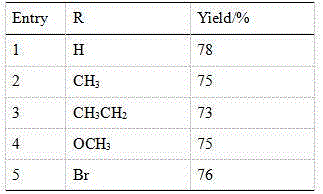
[0050] Synthesize the compound of the present invention according to the method for above-mentioned embodiment 1, when the substituting group R in the 4-substituted phenylacetylene is respectively H, CH 3 、CH 3 CH 2 , OCH 3 , Br, the productive rate of product is as follows:
[0051] Table 1:
[0052]
[0053] The following are the NMR data and mass spectrometry data of the five compounds:
[0054] 1. 2-(3,4-dimethoxy)benzoyl-5-(phenylethynyl)thiophene
[0055] Yield: 78%; mp102-103°C; 1HNMR (400MHz, CDCl3) δ7.63 (d, J=4.0Hz, 1H), 7.57 (m, 2H), 7.39 (m, 3H), 7.30 (d, J=4.0Hz, 1H), 7.25(d, J=8.0Hz, 1H), 7.18(d, J=8.0Hz, 1H), 7.16(s, 1H), 3.96(s, 3H), 3.94(s, [ M+H]+).
[0056] 2. 2-(3,4-dimethoxy)benzoyl-5-(4-methylphenylethynyl)thiophene
[0057] Productive rate: 75%; mp104-105 ℃; 1HNMR (400MHz, CDCl3) δ7.58 (d, J=3.6Hz, 1H), 7.40 (d, J=8.0Hz, 2H), 7.25 (d, J=8.0Hz, 2H) 8.0Hz, 1H), 7.24(d, J=3.6Hz, 1H), 7.18(d, J=8.0Hz, 1H), 7.14(d, J=8.0Hz, 2H), 7.12(s, 1H), 3.92...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com