Method for preparing solifenacin succinate
A technology of succinic acid and acid-binding agent, which is applied in the preparation of solifenacin succinate and the preparation of selective muscarinic M3 receptor antagonist drugs, and can solve the problem of unsuitable industrial application and high price of formyldiimidazole , the toxicity of raw material reagents and other problems, to achieve the effect of easy recycling, improving utilization rate and conversion rate, and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
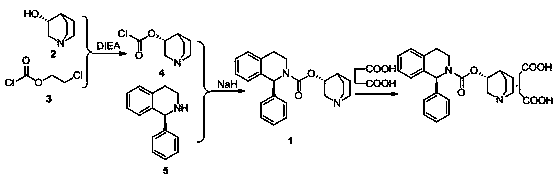
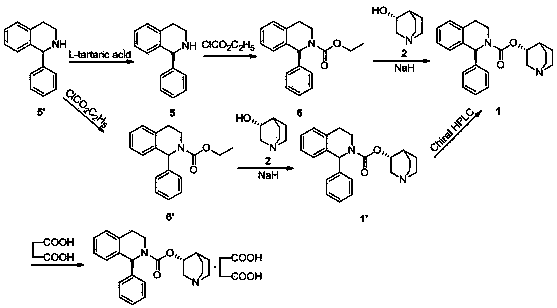
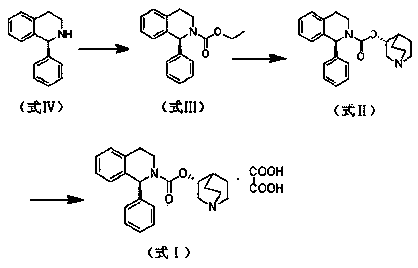
[0045] Sorenacin succinate ((3R)-1-azabicyclo[2.2.2]octane-3-yl (1S)-1-phenyl-3,4-dihydroisoquinoline-2 -(1H)-carboxylate succinate) preparation method, comprises the steps:
[0046]
Embodiment 1
[0048] 1) Add ionic liquid [bmim]BF in sequence to a 500ml three-neck bottle 4 250ml, 20.9g of formula IV compound (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline and 13.8g of potassium carbonate, under the condition of stirring, add 10.8g of chlorine dropwise to the reaction system After the addition of ethyl formate, react at room temperature for 2 hours. After the reaction, the reaction solution is first extracted three times with 50ml of dichloromethane, then washed with water three times, then dried with anhydrous sodium sulfate at room temperature for 2 hours, and finally distilled under reduced pressure. The dichloromethane was removed to obtain 27.0 g of an oily product, that is, compound (S)-1-phenyl-1,2,3,4-tetrahydroisoquinolinecarboxylic acid ethyl ester of formula III, and the yield was 96.1%.
[0049] 2) Add ionic liquid [bmim]BF in sequence to a 500ml three-neck bottle 4 250ml, formula III compound (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline carboxylic acid ethyl e...
Embodiment 2
[0053] 1) In a 500ml three-necked flask, add 250ml of toluene, 20.9g of the compound of formula IV (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline and 16.6g of potassium carbonate in sequence, under stirring conditions , add 10.8g of ethyl chloroformate dropwise to the reaction system. After the dropwise addition, react at room temperature for 2h. Sodium was dried at room temperature for 6 hours, and finally dichloromethane was distilled under reduced pressure to obtain 27.11 g of oily substance, namely the compound of formula III (S)-1-phenyl-1,2,3,4-tetrahydroisoquinolinecarboxylic acid ethyl Esters, the yield is 96.5%.
[0054] 2) Add ionic liquid [bmim]BF in sequence to a 500ml three-neck bottle 4 250ml, formula III compound (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline carboxylic acid ethyl ester 20g, DMF0.86g, (R)-3-quinine alcohol 11.7g and hydrogenation Sodium 0.5g, stirring and heating until the reaction system is 100°C, heat preservation reaction for 6h, after the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com