Detection analysis method for impurity homopiperazine in fasudil hydrochloride
The technology of an analysis method of fasudil hydrochloride is applied in the field of detection and analysis of the impurity homopiperazine in fasudil hydrochloride, which can solve the problems that the quality of the product is difficult to control and is not suitable for gas phase detection, etc., and achieves high accuracy, The effect of small harm and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
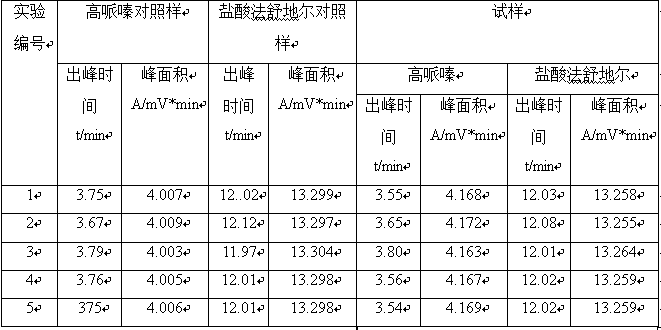
[0037] Determination according to ion chromatography (Chinese Pharmacopoeia 2010 edition two appendix Ⅴ J).
[0038] Chromatographic conditions and system suitability test Use carboxylic acid group-bonded silica gel as filler; 5mmol / L methanesulfonic acid aqueous solution-acetonitrile (90:10) as mobile phase; flow rate 1.0ml per minute; conductometric detection. Weigh each appropriate amount of fasudil hydrochloride and homopiperazine reference substance, dissolve and dilute with mobile phase to make a mixed solution containing about 3 mg of fasudil hydrochloride and 3 μg of homopiperazine in every 1 ml as the system suitability test solution, Precisely measure 20 μl and inject it into the liquid chromatograph, record the chromatogram, and the separation degree between the homopiperazine peak and the adjacent peak should meet the requirements.
[0039] Determination method Take about 0.3g of this product, weigh it accurately, put it in a 100ml measuring bottle, add mobile phas...
Embodiment 2
[0041] Determination according to ion chromatography (Chinese Pharmacopoeia 2010 edition two appendix Ⅴ J).
[0042] Chromatographic conditions and system suitability test Use carboxylic acid group-bonded silica gel as filler; 5mmol / L methanesulfonic acid aqueous solution-acetonitrile (75:25) as mobile phase; flow rate 0.3ml per minute; conductometric detection. Weigh each appropriate amount of fasudil hydrochloride and homopiperazine reference substance, dissolve and dilute with mobile phase to make a mixed solution containing about 3 mg of fasudil hydrochloride and 3 μg of homopiperazine in every 1 ml as the system suitability test solution, Precisely measure 20 μl and inject it into the liquid chromatograph, record the chromatogram, and the separation degree between the homopiperazine peak and the adjacent peak should meet the requirements.
[0043] Determination method Take about 0.3g of this product, weigh it accurately, put it in a 100ml measuring bottle, add mobile phas...
Embodiment 3
[0045] Determination according to ion chromatography (Chinese Pharmacopoeia 2010 edition two appendix Ⅴ J).
[0046] Chromatographic conditions and system suitability test Use carboxylic acid group bonded silica gel as filler; use 5mmol / L methanesulfonic acid aqueous solution-acetonitrile (95:5) as mobile phase; flow rate is 2ml per minute; conductivity detection. Weigh each appropriate amount of fasudil hydrochloride and homopiperazine reference substance, dissolve and dilute with mobile phase to make a mixed solution containing about 3 mg of fasudil hydrochloride and 3 μg of homopiperazine in every 1 ml as the system suitability test solution, Precisely measure 20 μl and inject it into the liquid chromatograph, record the chromatogram, and the separation degree between the homopiperazine peak and the adjacent peak should meet the requirements.
[0047]Determination method Take about 0.3g of this product, weigh it accurately, put it in a 100ml measuring bottle, add mobile pha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com