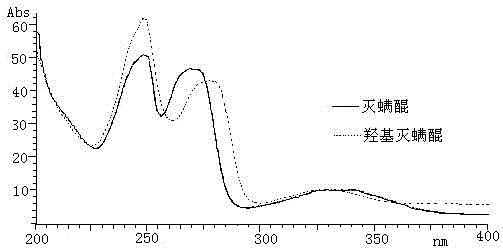
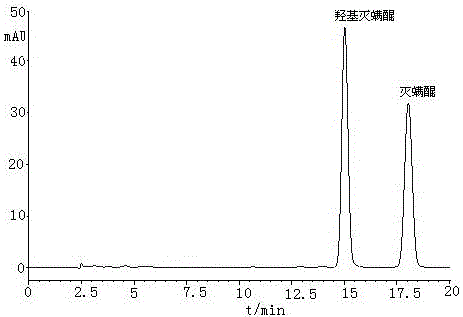
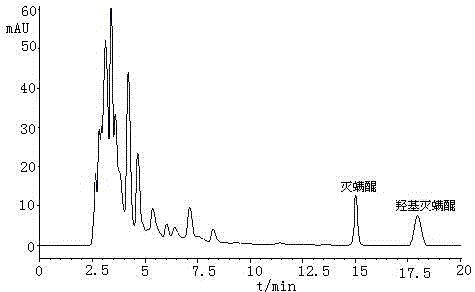
A method for determining acequinone and hydroxyacequinone using high performance liquid chromatography
A technology of high performance liquid chromatography and hydroxyfenacin, which is applied to instruments, measuring devices, scientific instruments, etc., can solve the problems of prolonging blood coagulation time, and achieve the effects of high accuracy, simple method and good reproducibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The present invention will be further described in detail below in conjunction with the accompanying drawings and specific embodiments.
[0024] In this embodiment, the instrument selected for the method of determining acequinone and hydroxyacequinone by using a high performance liquid chromatography is: a 1200 type high performance liquid chromatography with a diode array detector from Agilent Corporation of the United States; Model 2450 UV-Vis Spectrophotometer of Tianjin Company; BILONJJ-2 High-speed Tissue Breaker of Jiangsu Jintan Yitong Electronics Co., Ltd.; R-210 Rotary Evaporator of Swiss Buchi Co., Ltd.; 21011V001R200 of Swiss Buchi Company Nitrogen blowing instrument; TB215B electronic balance from Denver, USA; SyneryUV ultrapure water system from Millipore, USA.
[0025] The reagents used were: HPLC-grade n-hexane from MERCKMILLIPORE, Germany, HPLC-grade ethyl acetate from MERCKMILLIPORE, Germany, and HPLC-grade acetonitrile from Mallinckroat Baker; extracti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com