ELISA detection method for pipemidic acid and application thereof
A detection method, the technology of pipemidic acid, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of high detection limit, failure to meet the detection requirements of pipemidic acid, etc., achieve loss reduction, simple operation, and broad application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Synthesis and identification of pipemidic acid immunogen
[0066] 1. Weigh 5.2 mg of pipemidic acid and 20 mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC) and dissolve in 1 mL of phosphate buffer solution with 20 μL of sodium hydroxide , recorded as liquid A; weigh 15 mg of bovine serum albumin (BSA) and dissolve it in 1 mL of phosphate buffer, and record as liquid B; add liquid A into liquid B, stir at room temperature for 30-40 min, and fill the In the dialysis bag, dialyze with phosphate buffered saline solution at room temperature for 3 days, during which the dialysate is changed 6 times to obtain the target product pipemidic acid-BSA (immunogen).
[0067] 2. Identification of the immunogen
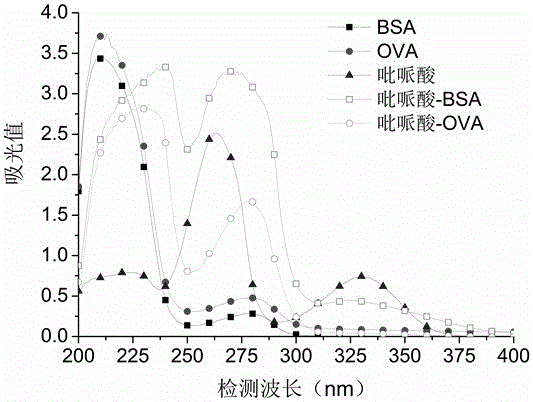
[0068] The pipemidic acid, BSA, and artificial antigen were scanned under ultraviolet light (200-400nm), and it was found that the absorption curve of the artificial antigen compared with BSA and pipemidic acid (see attached figure 1 Shown) have obvious ...
Embodiment 2
[0069] Synthesis and identification of embodiment 2 pipemidic acid coating former
[0070] 1. The general method is the same as in Example 1, but ovalbumin (OVA) is used as the carrier protein to obtain the target product pipemidic acid-OVA (enveloping source).
[0071] 2. Identification of the original coating
[0072] The pipemidic acid, OVA, and artificial antigen were scanned under ultraviolet light (200-400nm), and it was found that the absorption curve of the artificial antigen was higher than that of OVA and pipemidic acid (see attached figure 1 Shown) there is a significant change, it can be confirmed that the original coating was prepared successfully.
Embodiment 3
[0073] Synthesis and identification of embodiment 3 quinolones coating former
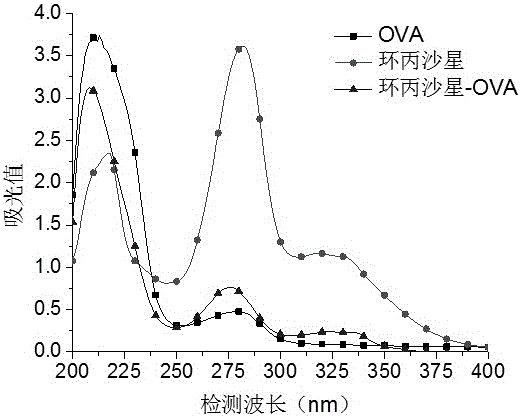
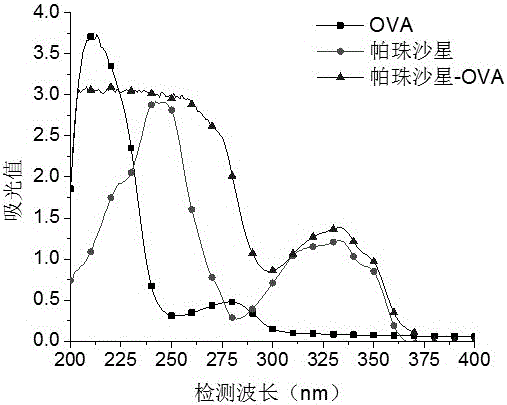
[0074] 1. Weigh 5.2 mg of quinolones (ciprofloxacin, pazufloxacin, gatifloxacin, lomefloxacin, sarafloxacin, and glycofloxacin) and dissolve them in 1 mL of phosphate buffer solution, and Add 15 mg ovalbumin (OVA). Add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide to the above solution, and stir at room temperature for 20-40 minutes. Put the reacted solution in a dialysis bag, dialyze with phosphate buffer solution at room temperature for 3 days, change the dialysate 6 times during the period, and obtain the target products ciprofloxacin-OVA, pazufloxacin-OVA, gatifloxacin-OVA, OVA, Lomefloxacin-OVA, Sarafloxacin-OVA, and Glinfloxacin-OVA.
[0075] 2. Identification of the original coating
[0076] The quinolones, OVA, and artificial antigens were scanned under ultraviolet light (200-400nm), and it was found that the absorption curves of artificial antigens compared with OVA and pipemidic acid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| reaction rate constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com