A kind of ELISA detection method and application of pipemidic acid
A detection method and technology of pipemidic acid, which are applied in measurement devices, instruments, scientific instruments and other directions, can solve the problems of failing to meet the detection requirements of pipemidic acid and high detection limit, and achieve the advantages of reducing loss, simple operation and broad application prospects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Synthesis and identification of pipemidic acid immunogen
[0066] 1. Dissolve 5.2 mg of pipemidic acid and 20 mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC) in 1 mL of phosphate buffer with 20 μL of sodium hydroxide solution, which is recorded as solution A; weigh 15 mg bovine serum albumin (BSA) and dissolve it in 1 mL phosphate buffer solution, and record it as solution B; add solution A to solution B, stir at room temperature for 30-40 min, and After the reaction, the solution was put into a dialysis bag, and dialyzed with phosphate buffered saline solution at room temperature for 3 days, during which the dialysate was changed 6 times to obtain the target product pipemidic acid-BSA (immunogen).
[0067] 2. Identification of the immunogen
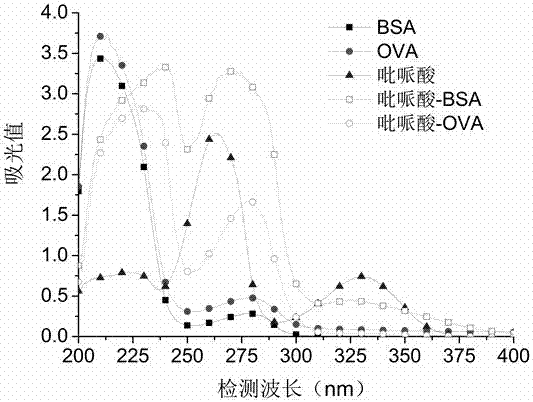
[0068] The pipemidic acid, BSA, and artificial antigen were scanned under ultraviolet light (200-400 nm), and it was found that the absorption curve of the artificial antigen was higher than that of BSA and pi...
Embodiment 2
[0069] Example 2 Synthesis and identification of pipemidic acid coating agent
[0070] 1. The general method is the same as in Example 1, but ovalbumin (OVA) is used as the carrier protein to obtain the target product pipemidic acid-OVA (enveloping source).
[0071] 2. Identification of the original coating
[0072] The pipemidic acid, OVA, and artificial antigen were scanned under ultraviolet light (200-400 nm), and it was found that the absorption curve of the artificial antigen was higher than that of OVA and pipemidic acid (see attached figure 1 Shown) there is a significant change, it can be confirmed that the original coating was prepared successfully.
Embodiment 3
[0073] Example 3 Synthesis and Identification of Quinolones Coating Progen
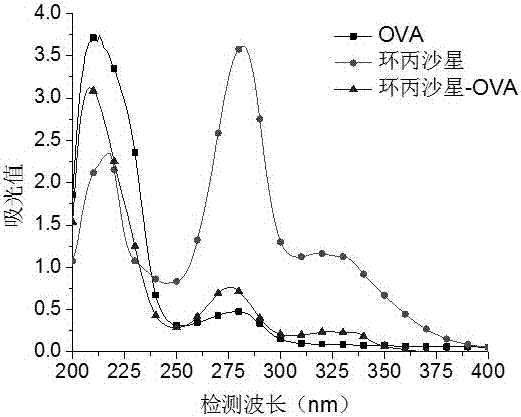
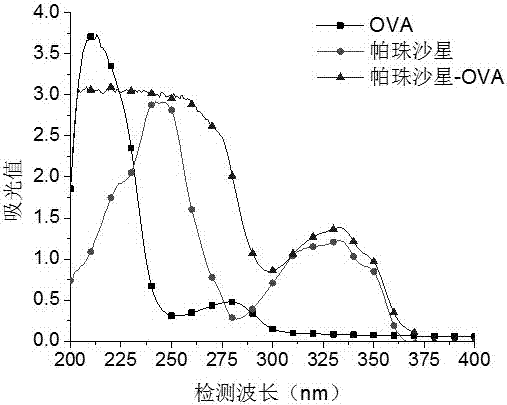
[0074] 1. Dissolve 5.2 mg of quinolones (ciprofloxacin, pazufloxacin, gatifloxacin, lomefloxacin, sarafloxacin, and glycofloxacin) in 1 mL of phosphate buffer solution, and Add 15 mg ovalbumin (OVA). Add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide to the above solution, and stir at room temperature for 20-40 min. Put the reacted solution in a dialysis bag, dialyze with phosphate buffer solution at room temperature for 3 days, change the dialysate 6 times during the period, and obtain the target products ciprofloxacin-OVA, pazufloxacin-OVA, gatifloxacin-OVA, OVA, Lomefloxacin-OVA, Sarafloxacin-OVA, and Glinfloxacin-OVA.
[0075] 2. Identification of the original coating
[0076] The quinolones, OVA, and artificial antigen were scanned under ultraviolet light (200-400 nm), and it was found that the absorption curve of the artificial antigen was higher than that of OVA and pipemidic acid (see attach...
PUM
| Property | Measurement | Unit |
|---|---|---|
| reaction rate constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com