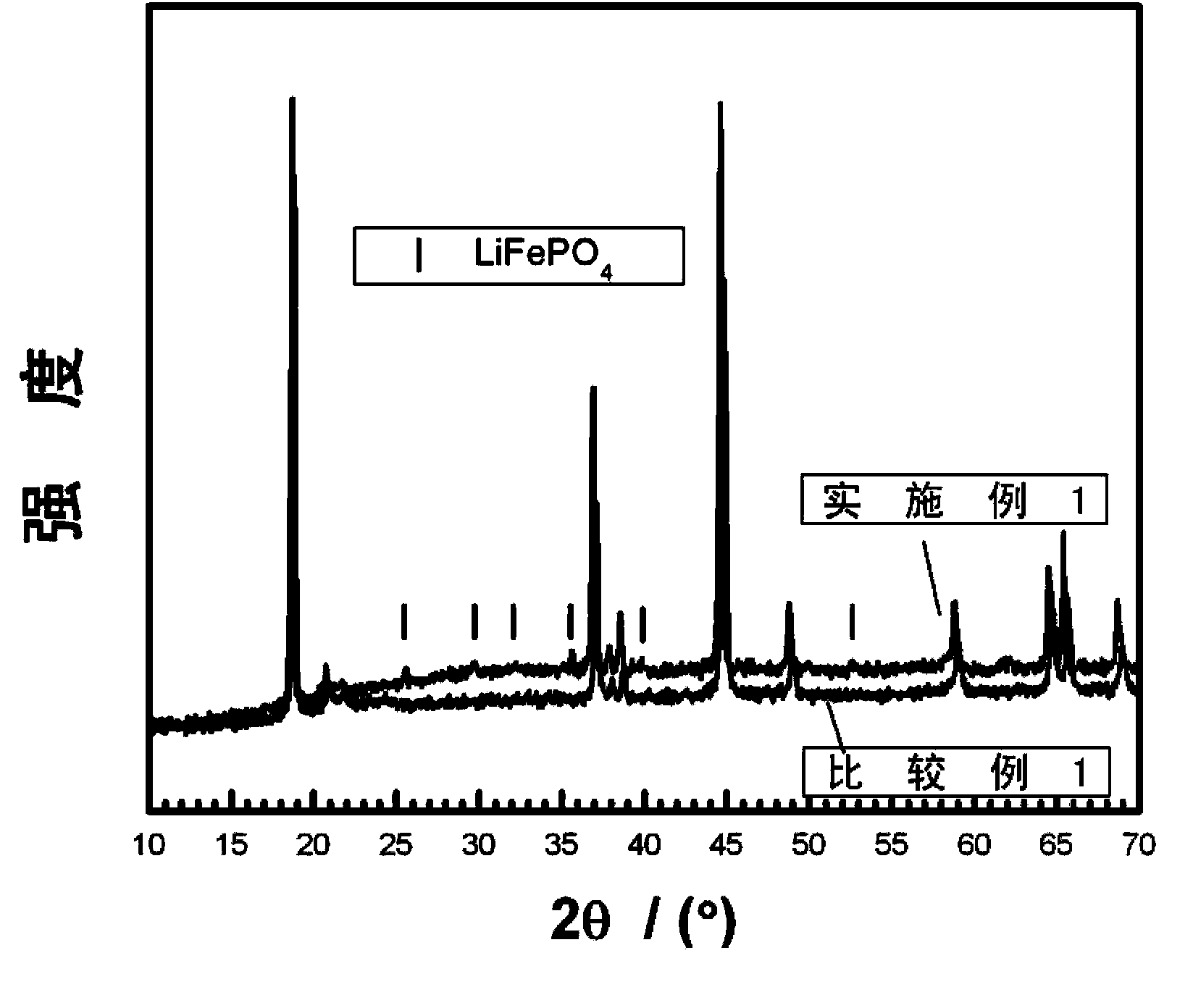
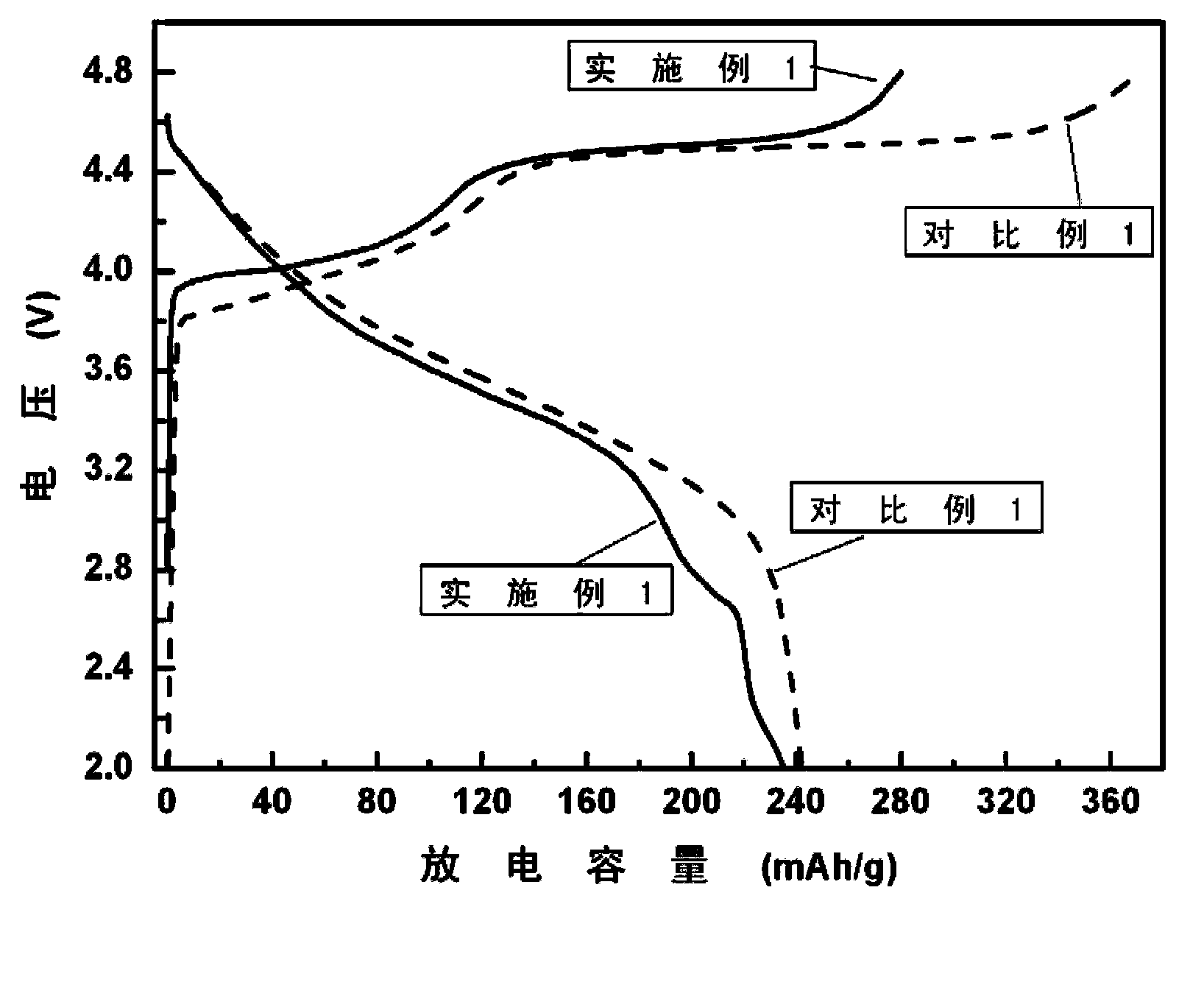
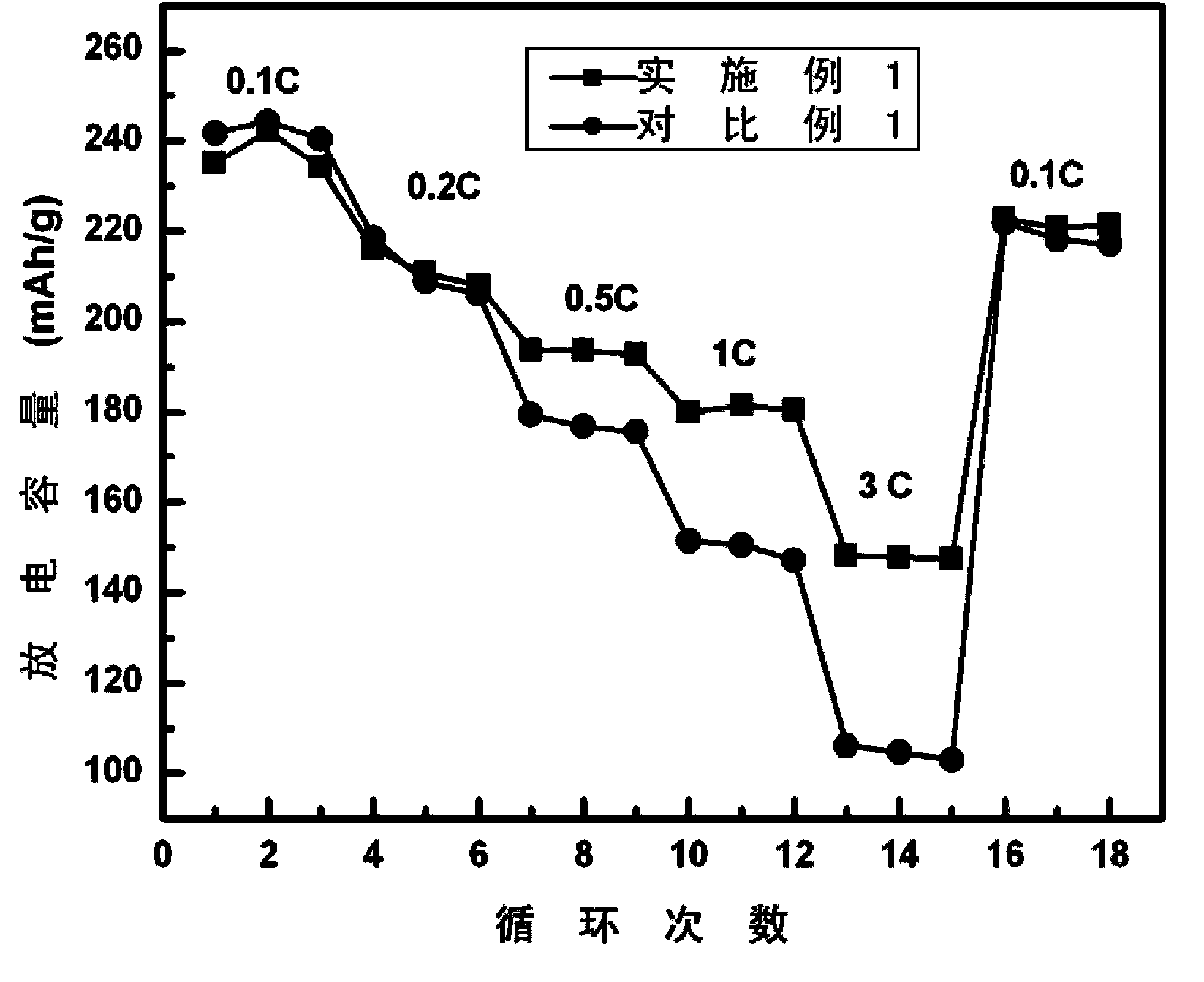
Surface coating and compounding lithium-rich manganese-based positive electrode material and preparation method of positive electrode material
A lithium-rich manganese-based, surface-coated technology, applied in battery electrodes, electrical components, electrochemical generators, etc., can solve the problems of poor rate performance, low initial charge and discharge efficiency, and achieve high charge and discharge efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Composite lithium-rich manganese-based cathode material Li[Li 0.15 Ni 0.13 co 0.13 mn 0.54 ]O 1.975 0.05LiFePO 4 (i.e. Li[Li x / 3-y Me 1-x mn 2x / 3 ]O 2-y / 2 ·yLiFePO 4 Where x=0.6, y=0.05, Me=Ni 0.325 co 0.325 mn 0.35 ).
[0039] 1) Li[Li 0.15 Ni 0.13 co 0.13 mn 0.54 ]O 1.975 0.05LiFePO 4 preparation of
[0040] Mix 5.68g nickelous oxide, 6.10g tricobalt tetroxide, 26.69g lithium carbonate, and 36.28g manganese carbonate, add deionized water according to 9 times the weight of the solid powder, and add it to the grinder for grinding until the particle size is less than 0.3 microns. Then spray-dry to obtain a mixed powder of 4 raw materials, calcined at 900°C for 36h, cool with the furnace, and then grind and sieve the powder to obtain Li[Li 0.2 Ni 0.13 co 0.13 mn 0.54 ]O 2 .
[0041] Weigh 2.02 g of ferric nitrate nonahydrate, 0.575 g of ammonium dihydrogen phosphate and 0.24 g of glucose dissolved in 80 mL of water, weigh 8.64 g of the above Li[Li...
Embodiment 2-5
[0047] Example 2-5: A series of lithium-rich manganese-based positive electrode materials coated on the surface Li[Li 0.2-y Ni 0.13 co 0.13 mn 0.54 ]O 2-y / 2 ·yLiFePO 4 (i.e. Li[Li x / 3-y Me 1-x mn 2x / 3 ]O 2-y / 2 ·yLiFePO 4 where x=0.6, Me=Ni 0.325 co 0.325 mn 0.35 ). The value of y is shown in Table 1.
[0048] The preparation method of the material is the same as that provided in Example 1, and the ratio of the corresponding raw materials is calculated according to the chemical formula in this example.
[0049] [Table 1]
[0050] Example
Embodiment 6
[0051] Embodiment 6: the lithium-rich manganese-based cathode material of surface coating composite: Li[Li 0.113 Ni 0.195 co 0.195 mn 0.477 ]O 1.99 0.02LiFePO 4 (i.e. Li[Li x / 3-y Me 1-x mn 2x / 3 ]O 2-y / 2 ·yLiFePO 4 Where x=0.4, y=0.02, Me=Ni 0.325 co 0.325 mn 0.35 ).
[0052] 8.2g nickelous oxide, 8.8g tricobalt tetroxide, 24.21g lithium carbonate, 30.80g manganese carbonate are mixed, add deionized water by 19 times of solid powder weight, join in grinder and grind, until medium particle size is less than 0.3 micron, then Carry out spray drying to obtain the mixed powder of 4 kinds of raw materials, calcinate 25h through 850 ℃, cool with furnace, then powder is ground and sieved to obtain Li[Li 0.133 Ni 0.195 co 0.195 mn 0.477 ]O 2 .
[0053] Weigh 0.808 ferric nitrate nonahydrate, 0.23g ammonium dihydrogen phosphate and 0.12g glucose are dissolved in 80mL water, weigh 8.64g of the above-mentioned Li[Li 0.133 Ni 0.195 co 0.195 mn 0.477 ]O 2 Put it into t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com