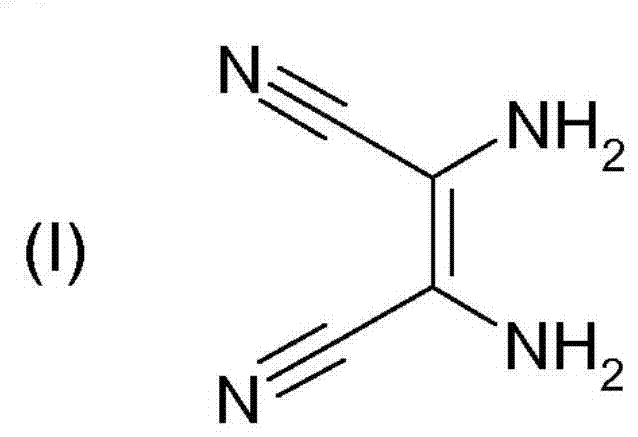
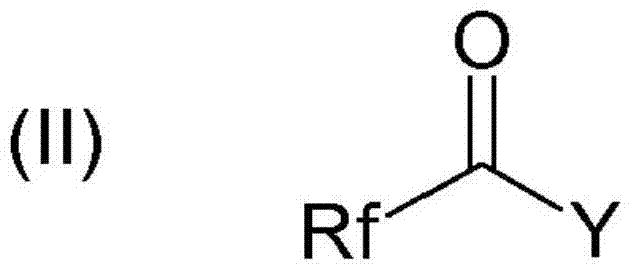
Method for preparing pentacyclic anion salt
A technology of salinization and carbon atom is applied in the field of 1-trifluoromethyl-4,5-dicarbonitrile-imidazolyl lithium to prepare electrolyte compositions containing this salt, which can solve problems such as safety and achieve good The effect of yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
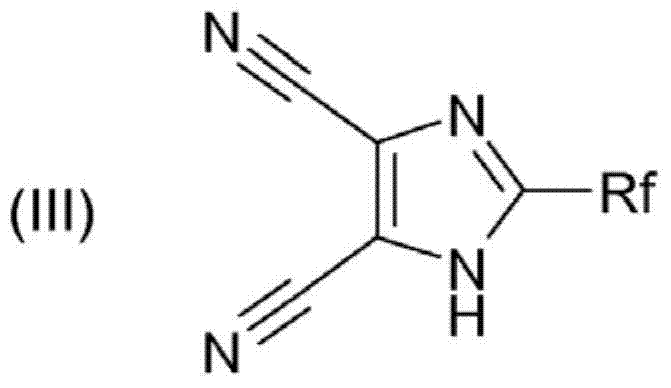
[0062] Preparation of imidazolyllithium
[0063] The imidazolyllithium of the following formula is prepared from the imidazole compound of (III) by reacting the imidazole compound of formula (III) with a lithium base:
[0064]
[0065] The lithium base is preferably selected from lithium hydride, lithium carbonate and lithium hydroxide and combinations thereof.
[0066] For example, when the imidazole compound has been isolated and purified as described above after the reaction, the obtained organic phase can be extracted with an aqueous solution of a lithium base. The aqueous phase can then be evaporated (after optional treatment with activated carbon).
[0067] The organic phase thus contains compound (III) together with the acid catalyst and remaining YH dissolved in the reaction solvent. Then, compound (III) is at a concentration of preferably 0.01 to 5 mol / L and preferably 0.1 to 3 mol / L. The concentration of the lithium base in the aqueous phase is preferably 0.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com