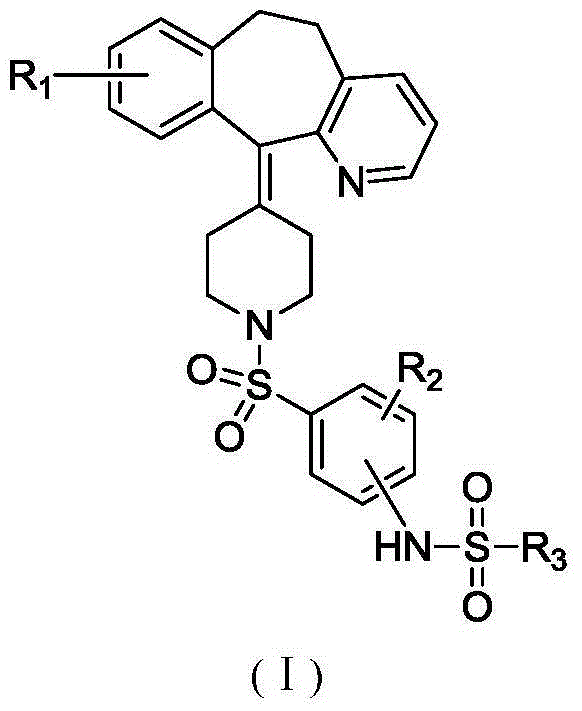
Bis-sulfonamide compound and its preparation method and use
A compound and pharmaceutical technology, which is applied in its preparation method and in the fields of medicine, bissulfonamide compounds, and medicine, can solve the problems of benzazepine compound activity and insufficient physical and chemical properties of side effects, and achieve obvious antagonistic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055]
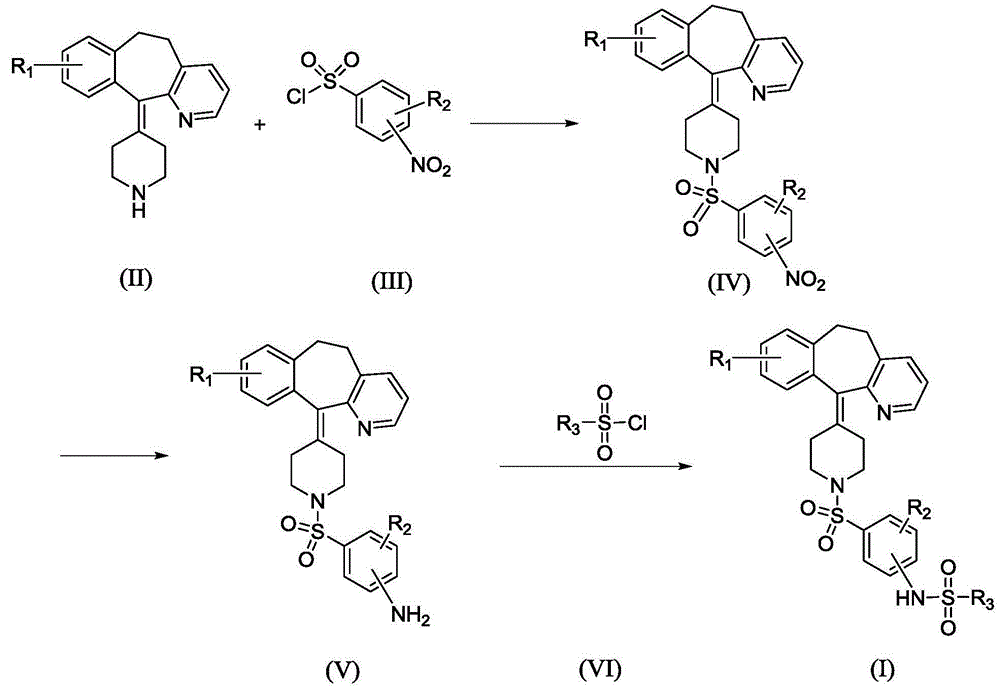
[0056] II-1 (50g, 161mmol) is placed in a 1000mL reaction flask, and CH is added 2 Cl 2 (300mL) Stir to dissolve, add triethylamine (49g, 483mmol), stir at room temperature, add Intermediate III-1 (35.7g, 161mmol) in batches, keep the temperature and stir for 6h, TLC detection shows that the reaction is complete ( Developing agent ethyl acetate: petroleum ether=1:3).
[0057] Pour the reaction solution into 200 ml of cold water, fully shake the layer, divide the organic layer, and wash with water three times in this way. The organic layer was dried with anhydrous sodium sulfate and left overnight. Filter and evaporate the solvent under reduced pressure to obtain a crude light yellow solid. The obtained crude product was recrystallized from ethanol to obtain 77.6 g of white solid. The purity is 98.5% (HPLC normalization method), and the yield is 97.2%. ESI-MS: 496.1.
Embodiment 2
[0059]
[0060]
[0061] Place II-1 (50g, 161mmol) in a 250mL reaction flask, add pyridine (150mL), stir to dissolve, stir at -5°C, add Intermediate III-2 (35.7g, 161mmol) in batches, keep stirring at temperature 12h, TLC detection showed that the reaction was over (developing solvent ethyl acetate: petroleum ether = 1:3).
[0062] The reaction liquid was poured into 4500 ml of cold water and stirred, and a solid precipitated out. Filter, wash the filter cake with water, and dry to obtain a crude dark yellow solid. This crude product was recrystallized from ethanol to obtain 76.7 g of a yellow solid. The purity is 98.9% (HPLC normalization method), and the yield is 96.0%. ESI-MS: 496.1.
Embodiment 3
[0064]
[0065] II-2 (20g, 72mmol) is placed in a 250mL reaction flask, and CHCl is added 3 (100mL) Stir to dissolve, add pyridine (11.4g, 144mmol), stir at 60℃, add Intermediate III-3 (17.1g, 72mmol) in batches, keep the temperature and stir for 5h, TLC detection shows that the reaction is complete (expanded Ethyl acetate: petroleum ether = 1:3).
[0066] The reaction solution was poured into 100 ml of cold water, fully shaken to separate into layers, and the organic layer was separated and washed three times in succession. The organic layer was dried with anhydrous sodium sulfate and left to stand overnight. Filter and evaporate the solvent under reduced pressure to obtain a crude light yellow solid. The obtained crude product was purified by silica gel column chromatography to obtain 26.8 g of white solid. The purity is 99.1% (HPLC normalization method), and the yield is 78.2%. ESI-MS: 476.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com