Method for preparing lithium-nickel-cobalt-aluminium oxide material by adopting low-heat solid-phase reaction
A lithium-nickel-cobalt-aluminum, low-heat solid-phase technology is applied in the field of preparing lithium-nickel-cobalt-aluminum oxide cathode materials by low-heat solid-phase reaction, which can solve the problems of high energy consumption required, high reaction temperature, difficult to mix uniformly, etc. Production cost, the effect of reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) LiOH·H 2 O (12.445g), Ni(NO 3 ) 2 ·6H 2 O (69.790g), Co (NO 3 ) 2 ·6H 2 O (13.096g), Al(NO 3 ) 3 9H 2 O (5.627g) was weighed according to the molar ratio (1.06:0.8:0.15:0.05), and mixed in a high-speed mixer at a speed of 2000 rpm. After mixing for 15 minutes, a green paste precursor was obtained. It was vacuum-dried at 120° C. for 12 h.
[0027] (2) Calcining the prepared precursor in an oxygen or air atmosphere at 700°C for 8 hours to obtain the final product.
Embodiment 2
[0029] (1) LiOH·H 2 O (12.445g), Ni(NO 3 ) 2 ·6H 2 O (69.790g), Co (NO 3 ) 2 ·6H 2 O (13.096g), Al(NO 3 ) 3 9H 2 O (3.939g), MgCl 2 ·6H 2 O (0.919g) was weighed according to the molar ratio (1.06:0.8:0.15:0.035:0.015), and mixed in a high-speed mixer with a speed of 2000 rpm. After mixing for 15 minutes, a green paste precursor was obtained . It was vacuum-dried at 120° C. for 12 h.
[0030] (2) Calcining the prepared precursor in an oxygen or air atmosphere at 700°C for 8 hours to obtain the final product.
Embodiment 3
[0032] (1) LiOH·H 2 O (12.445g), Ni(NO 3 ) 2 ·6H 2 O (69.790g), Co (NO 3 ) 2 ·6H 2 O (13.096g), Al(NO 3 ) 3 9H 2 O (3.939g), H 3 BO 3 (0.278g) weighed by molar ratio (1.06:0.8:0.15:0.035:0.015), mixed in a high-speed mixer with a mixer speed of 2000 rpm, and after mixing for 15 minutes, a green paste precursor was obtained. It was vacuum-dried at 120° C. for 12 h.
[0033] (2) Calcining the prepared precursor in an oxygen or air atmosphere at 700°C for 8 hours to obtain the final product.
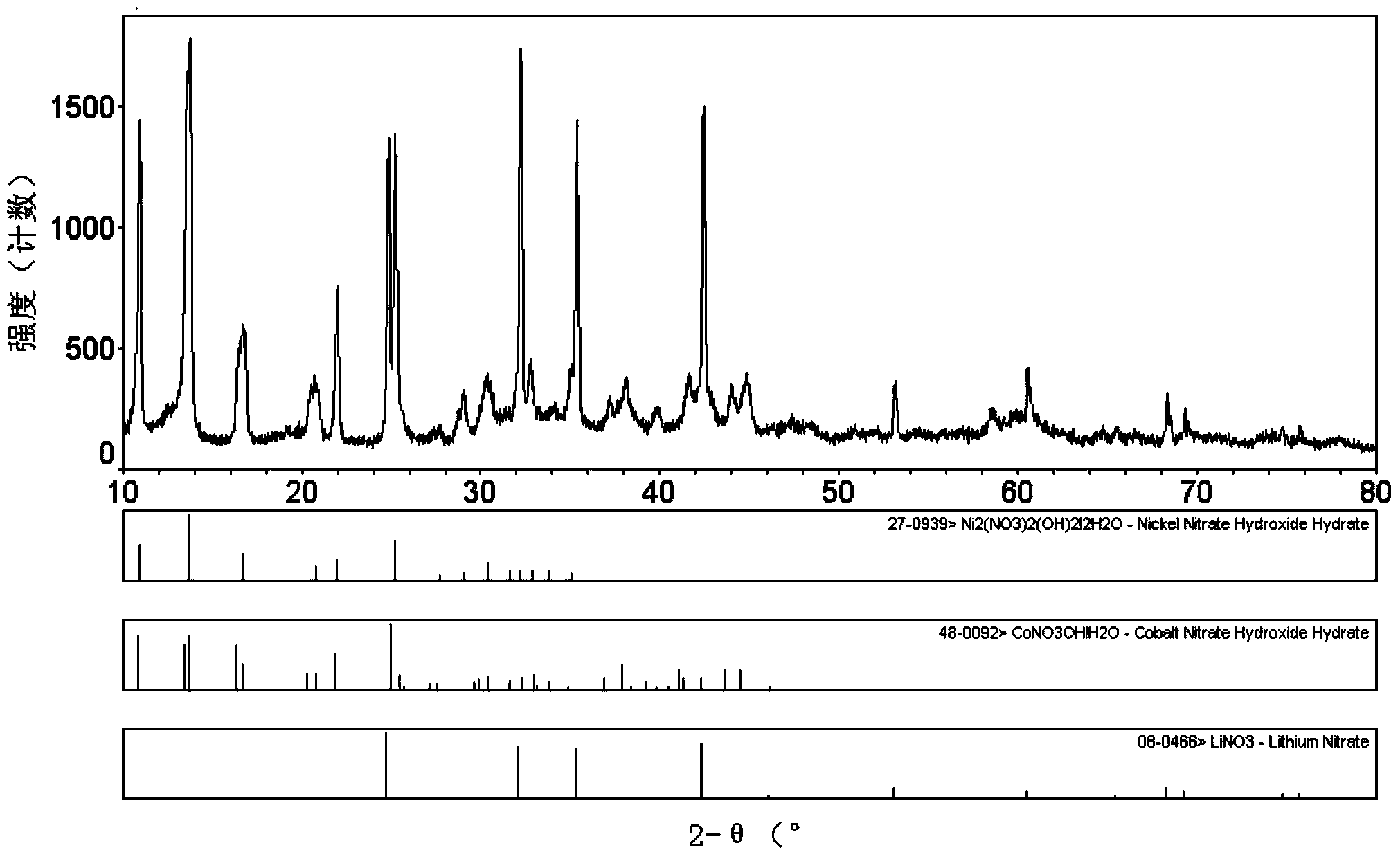
[0034] From figure 1 The XRD pattern of the medium precursor shows that when the precursor is prepared by high-speed mixing, most of the crystal water has been removed, and the hydroxyl groups in LiOH are broken off and combined with Ni, Co, etc. to form metal hydroxides, while Li + with NO 3 - combined to form LiNO 3 .
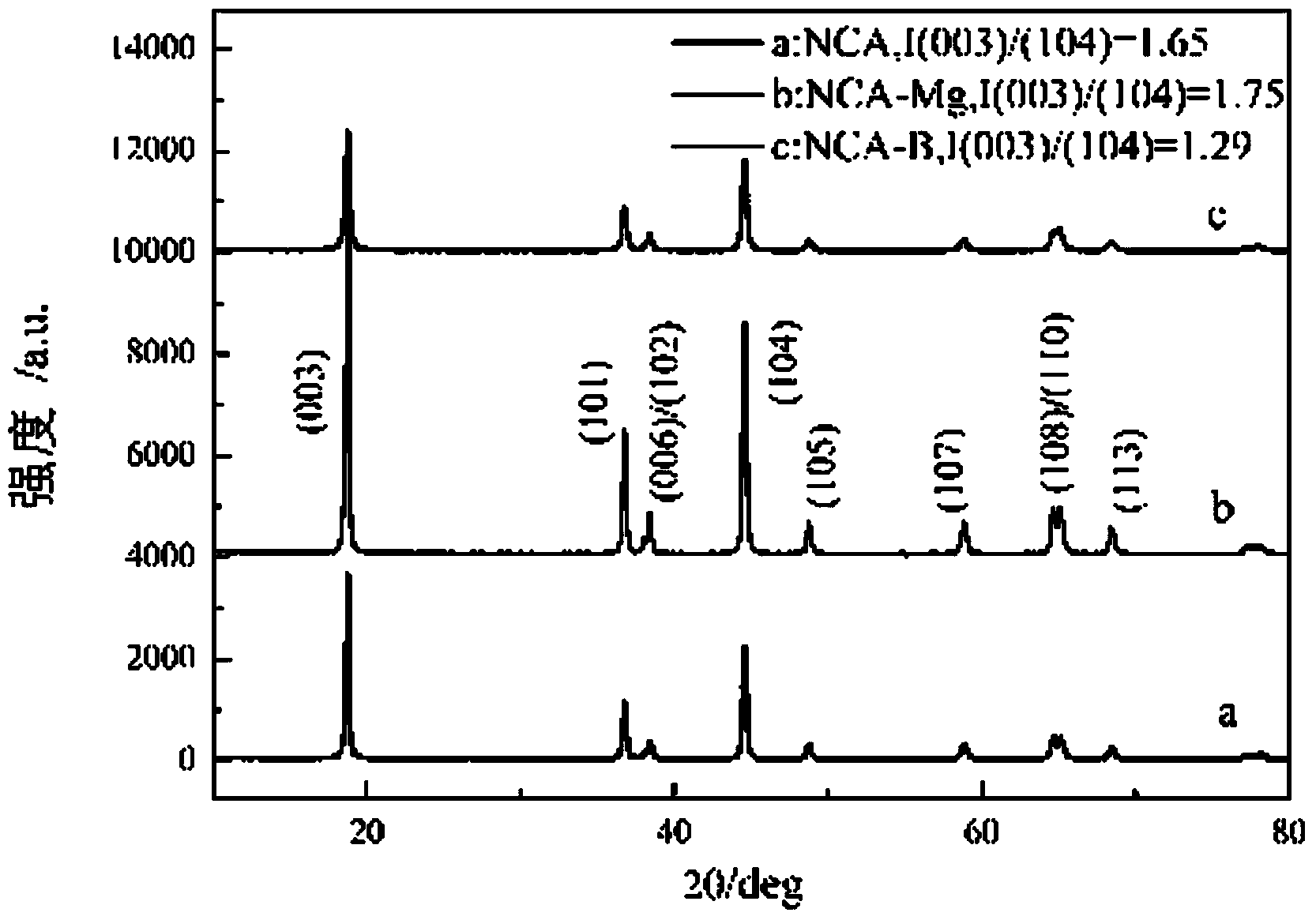
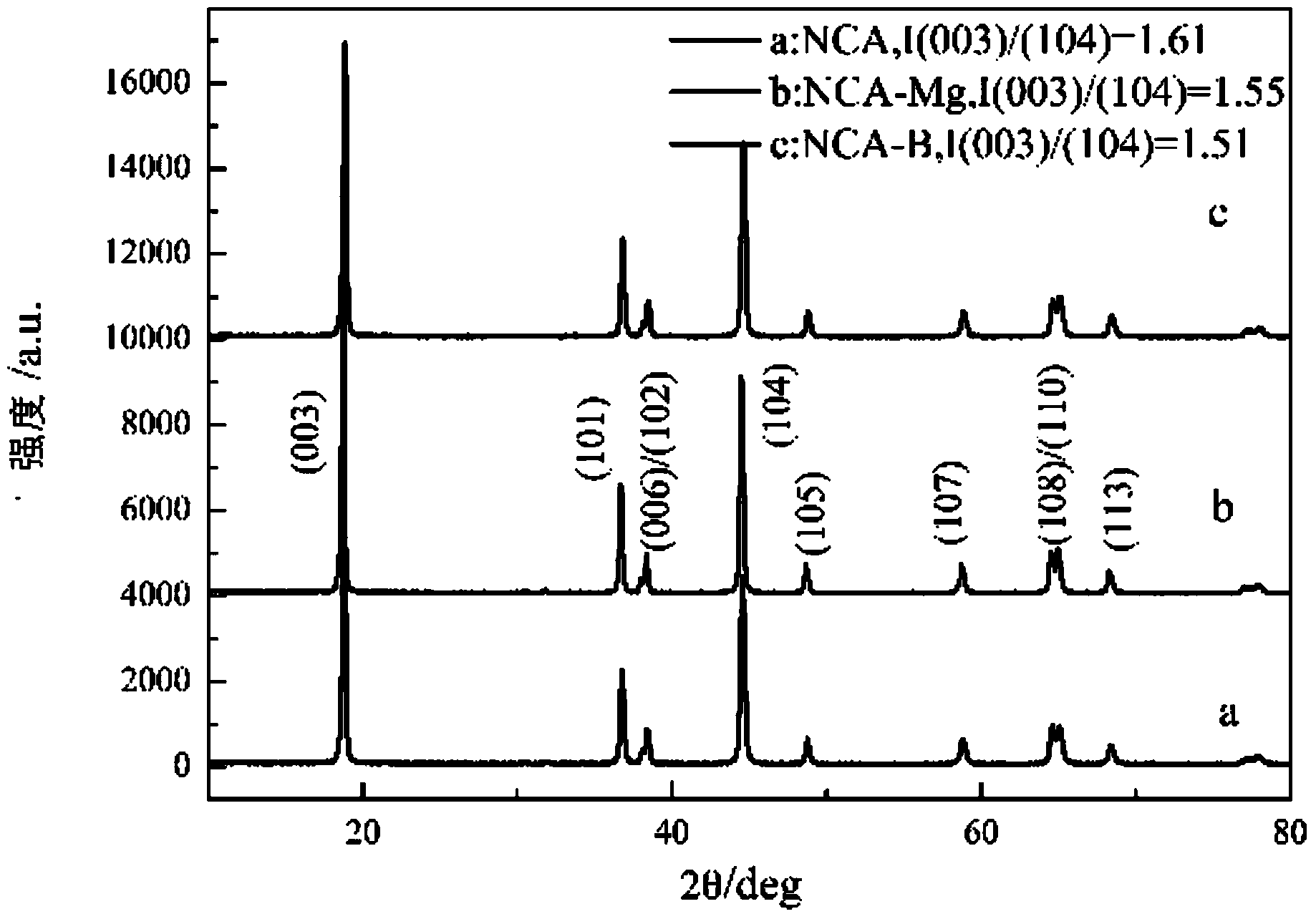
[0035] From figure 2 , image 3 It can be seen that the crystallization of the nickel-cobalt-aluminate lithium sample obtained by the low-temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com