Arborescence half antigen capable of directly detecting furaltadone metabolite AMOZ, arborescence antigen and application of arborescence half antigen
A technology of furaltadone and hapten, which is applied in the field of dendritic hapten to achieve the effects of high sensitivity, enhanced immunogenicity and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of embodiment 1 AMOZ glyoxylic acid derivative I:
[0046] Take 604mg (3mmol) of AMOZ and 266.5mg (3.6mmol) of glyoxylic acid and dissolve them in 2mL of absolute ethanol respectively, add the ethanol solution of glyoxylic acid to the ethanol solution of AMOZ dropwise during the stirring process, and stir at room temperature overnight. After the reaction was completed, vacuum filtration was performed, and the filter cake was washed twice with 2 mL of ethanol and diethyl ether, and the precipitate was collected to obtain 540.2 mg of the target product as a white solid with a yield of 70%. ESI-MS analysis (positive) m / z 258.7 [M+H] + ; 1 H NMR (600MHz, d5-Pyridine, TMS): δ 7.56 (s, 1H), 4.99-4.97 (m, 1H), 4.16 (t, J=8.8Hz, 1H), 3.79 (dd, J1=6.7, J2 =8.8, 1H), 3.68-3.64 (m, 4H), 2.66 (dd, J1=6.1, J2=13.5, 1H), 2.62 (dd, J1=5.5, J2=13.4, 1H), 2.51-2.46 (m , 4H). The structural formula of AMOZ glyoxylic acid derivative I is shown in formula (I).
Embodiment 2
[0047] The preparation method of embodiment 2 four-branched polyamide-amine II
[0048] Ethylenediamine and methyl acrylate are used as raw materials, and the reaction follows Michael addition and amidation condensation reactions. With ethylenediamine as the core, one terminal amino group is protected by tert-butoxycarbonyl (Boc), and Michael addition reaction is carried out with methyl acrylate to obtain Boc-Eddp-OCH 3 ; One terminal amino group of ethylenediamine is protected with fluorenylmethoxycarbonyl (Fmoc), and Boc-Eddp-OCH3 is subjected to amidation condensation reaction with excess Fmoc-protected ethylenediamine to obtain Boc-Eddp-(Fmoc-Ethylenediamine) 2 , go to Boc protection to get H-Eddp-(Fmoc- Ethylenediamine) 2 ; H-Eddp-(Fmoc- Ethylenediamine) 2 Further with Boc-Eddp-OCH 3 Carry out amidation condensation reaction, obtain Boc-Eddp-(Eddp) 2 -(Fmoc-Ethylenediamine) 4 , go to Fmoc protection to get Boc-Eddp-(Eddp) 2 -(Ethylenediamine) 4 , that is, the f...
Embodiment 3
[0049] Example 3 Preparation method of AMOZ dendritic hapten HⅢ
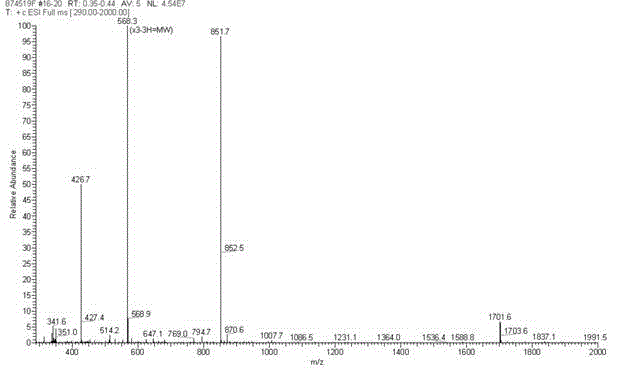
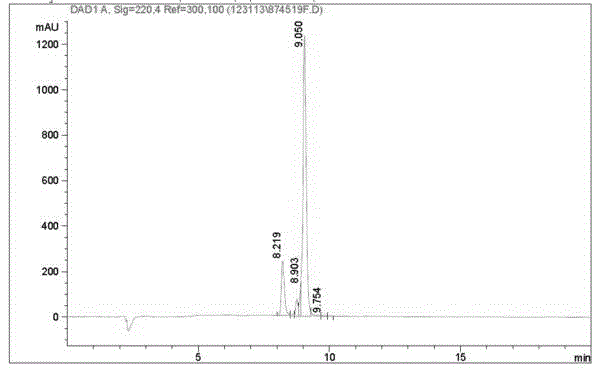
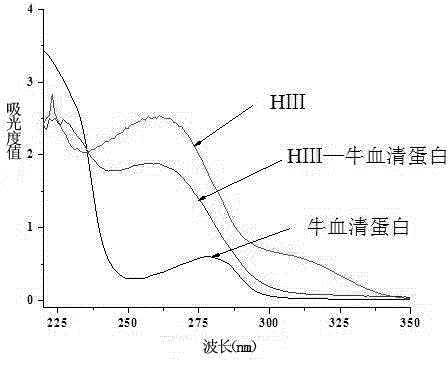
[0050] The four-branched polyamide-amine prepared in Example 2 and the glyoxylic acid derivative of AMOZ prepared in Excessive Example 1 were subjected to amidation condensation reaction to obtain Boc-Eddp-(Eddp) 2 -(AMOZ-2-iminoacetyl-Ethylenediamine) 4 , remove the Boc amino protecting group to obtain Eddp-(Eddp) 2 -(AMOZ-2-iminoacetyl-Ethylenediamine) 4 , the target AMOZ dendritic hapten HⅢ. The structure was identified by ESI-MS, and the purity was determined by HPLC. The structural formula of AMOZ dendritic hapten HIII is shown in formula (III).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com