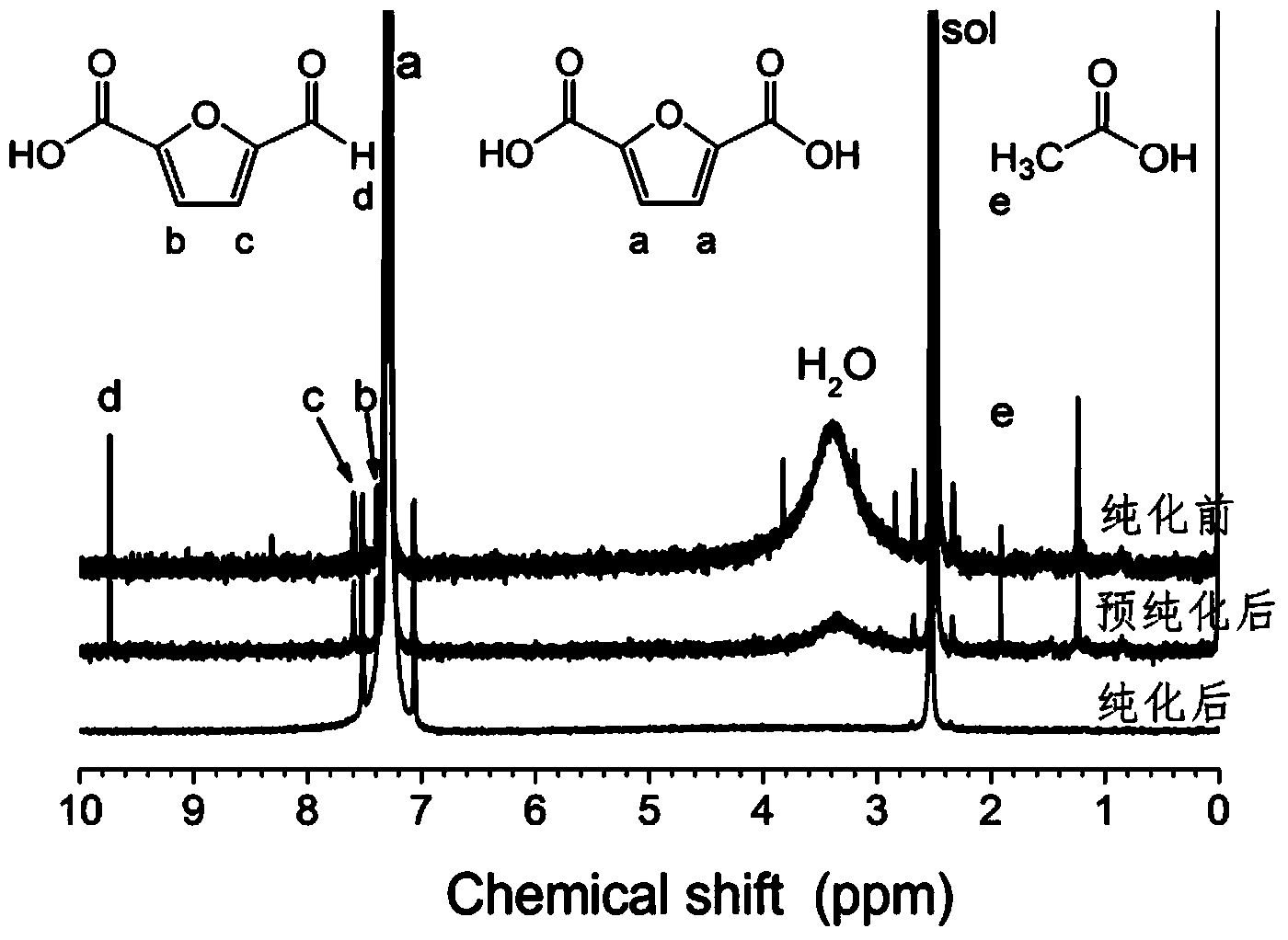
Method for purifying furandicarboxylic acid
A technology of furandicarboxylic acid and furandicarboxylate, applied in the direction of organic chemistry and the like, can solve the problems of long reaction time, application limitation, cumbersome process, etc., and achieve the effects of reducing yellowing phenomenon, improving molecular weight, and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
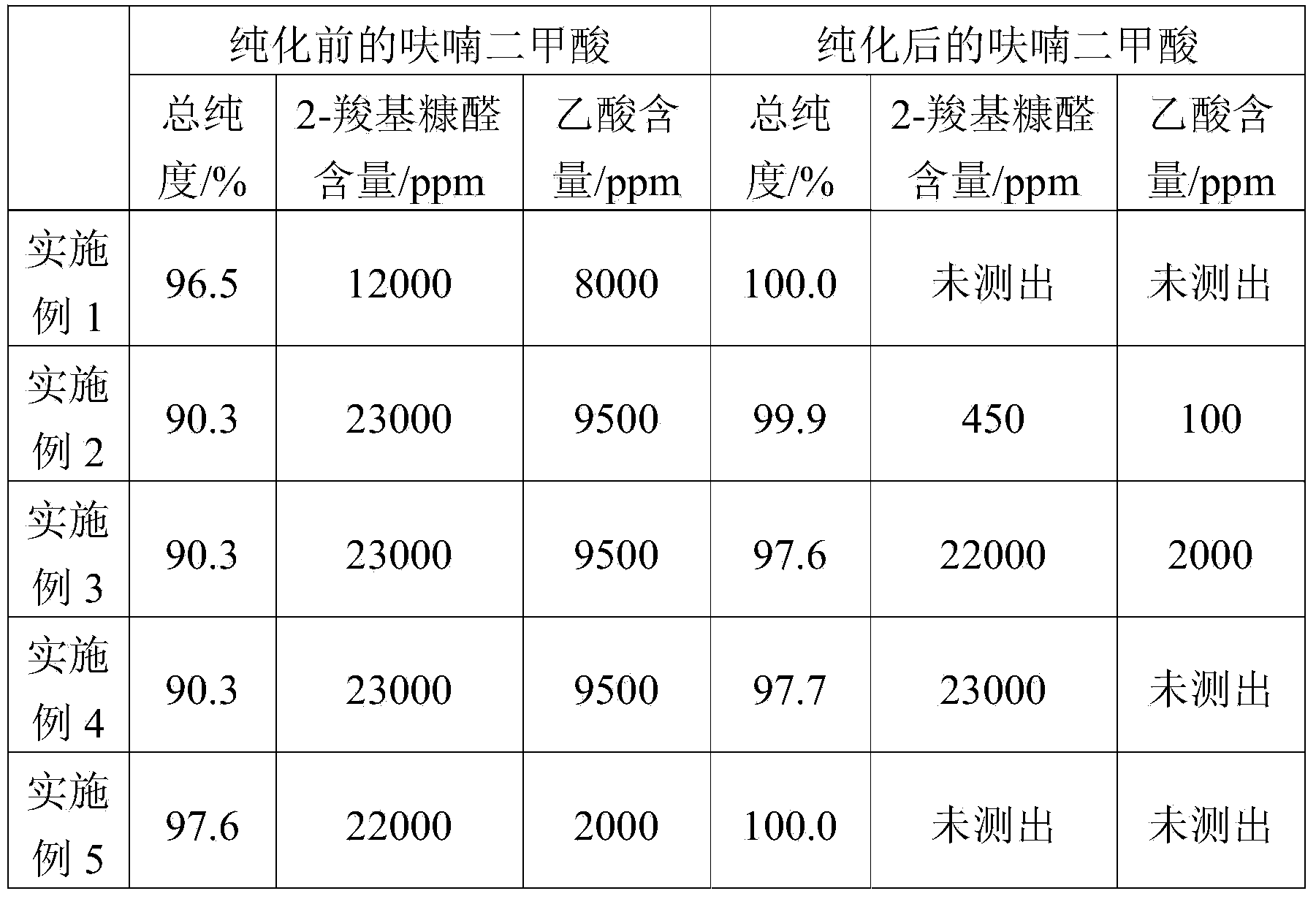
[0048] (1) Add 12 grams of sodium hydroxide, 20 grams of furandicarboxylic acid to be purified (purity 96.5%, wherein 2-carboxyfurfural content 12000ppm, acetic acid content 8000ppm) and 1 gram of activated carbon into water, fully stir to make the solid matter completely Dissolved to obtain an aqueous solution of furandicarboxylic acid salt.
[0049] (2) After filtering the furandicarboxylic acid salt aqueous solution, add concentrated hydrochloric acid to the filtrate under stirring to make the pH of the solution = 2, and obtain a solid precipitate.
[0050] (3) Filter out the solid precipitate, wash with water, and dry to obtain high-purity furandicarboxylic acid.
[0051] After testing, no impurities remain in the purified high-purity furandicarboxylic acid, and its purity is 100.0%.
Embodiment 2
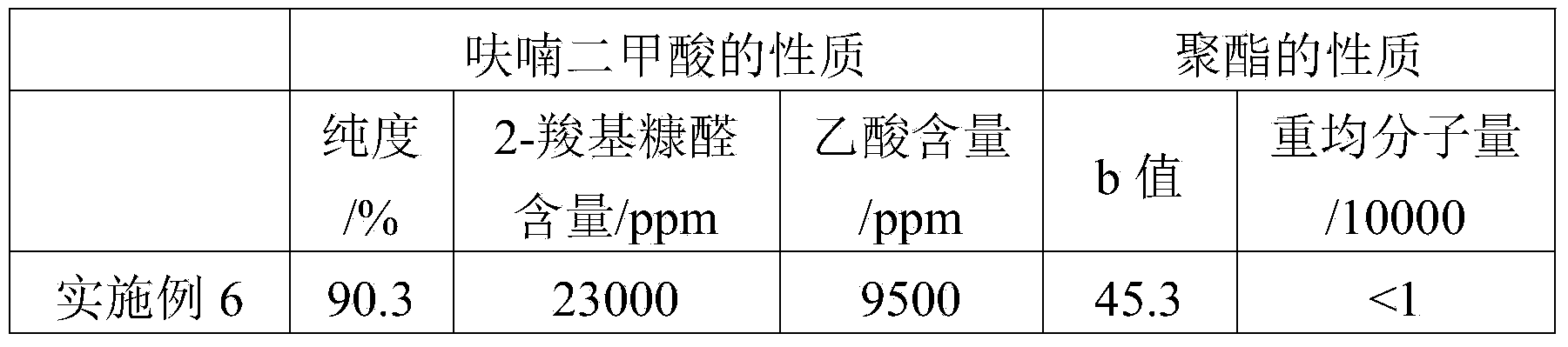
[0053] The method of Example 1 was repeated. The difference is that the raw material is furandicarboxylic acid to be purified (purity 90.3%, 2-carboxyfurfural content 23000ppm, acetic acid content 9500ppm).
[0054] After testing, the purified high-purity furandicarboxylic acid has a purity of 99.9%, in which the content of 2-carboxyfurfural is 450 ppm and the content of acetic acid is 100 ppm.
Embodiment 3
[0056] (1) 100 grams of furandicarboxylic acid to be purified (purity 90.3%, wherein 2-carboxyfurfural content 23000ppm, acetic acid content 9500ppm) was mixed with 500 grams of acetic acid, and stirred for 240 minutes under heating and reflux;
[0057] (2) Filter out the solid insolubles and wash with water to obtain the purified furandicarboxylic acid.
[0058] After testing, the purified furandicarboxylic acid has a purity of 97.6%, wherein the content of 2-carboxyfurfural is 22000ppm, and the content of acetic acid is 2000ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com