Application of gold nanoparticles in preparing anticoagulants or antiplatelet drugs
A gold nanoparticle and antiplatelet agent technology, applied in the field of biomedicine, can solve problems such as complex synthesis process, and achieve the effects of simple preparation method, high efficiency, small side effects, and small particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
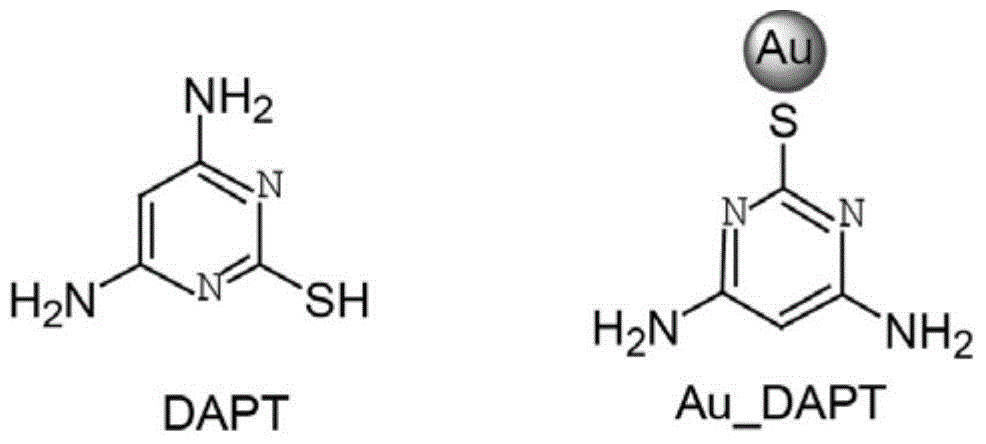
[0027] Example 1 Preparation of substituted pyrimidine-modified gold nanoparticles Au_DAPT
[0028] (1) Configure the reaction solution
[0029] Configure 5mM chloroauric acid methanol solution: dissolve 41mg chloroauric acid in 20mL anhydrous methanol;
[0030] Prepare 10 mM 2-mercapto-4,6-diaminopyrimidine solution: add 14 mg of 2-mercapto-4,6-diaminopyrimidine (DAPT) into 10 mL of anhydrous methanol, and add 200 μL of anhydrous acetic acid and 50 μL of Tween 80, ultrasonic dissolution;
[0031] Prepare 60mM sodium borohydride methanol solution: dissolve 12mg sodium borohydride in 3mL methanol, ready to use;
[0032] (2) Reduction reaction to prepare gold nanoparticles
[0033] Under an ice bath, mix 20 mL of chloroauric acid methanol solution and 10 mL of 2-mercapto-4,6-diaminopyrimidine solution into a 50 mL round bottom flask, and stir for 10 min with a magnet;
[0034] Increase the stirring speed, add sodium borohydride methanol solution dropwise, drop it within 2 mi...
Embodiment 2
[0037] Preparation of Example 2 Substituted Pyrimidine Modified Gold Nanoparticles Au_APT and Au_DHPT
[0038] The synthesis method of Au_APT (APT is 2-mercapto-4-aminopyrimidine) and Au_DHPT (DHPT is 2-mercapto-4,6-dihydroxypyrimidine) is similar to Au_DAPT, just replace DAPT with equimolar APT and DHPT.
Embodiment 3
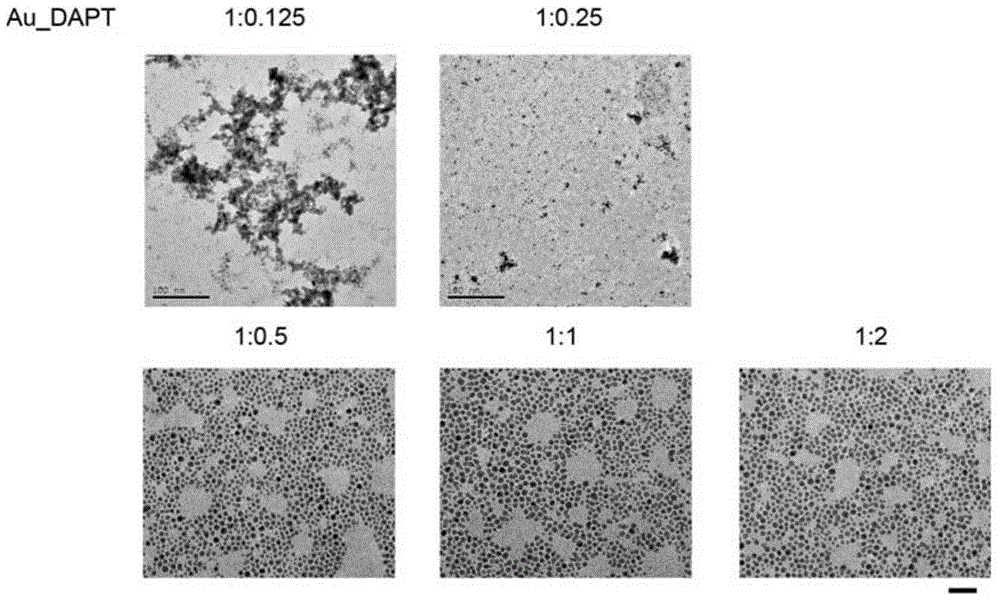
[0039] Example 3 Investigation of Gold Nanoparticles with Different Molar Content Ratio of Gold Elements and Substituted Pyrimidine Molecules
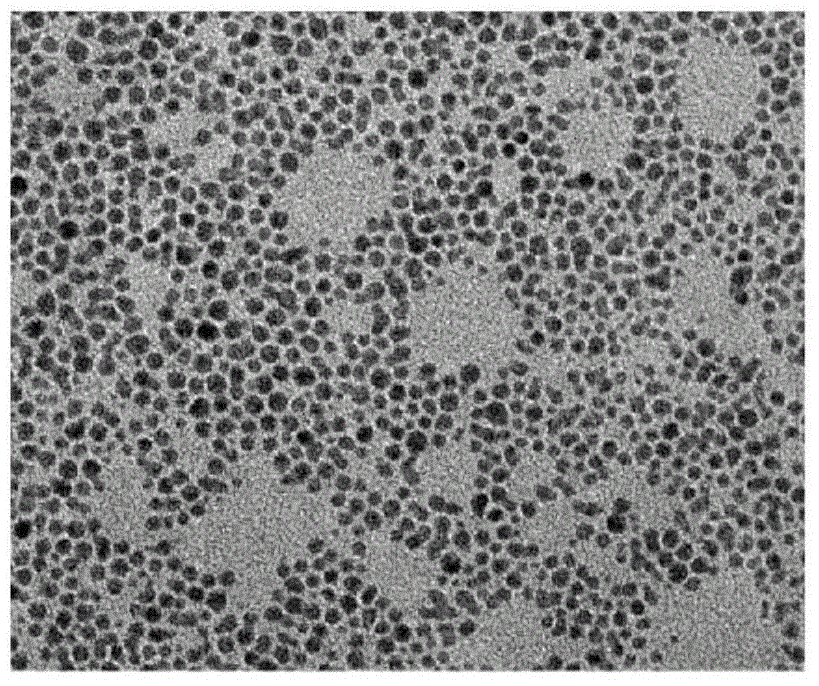
[0040] The molar content ratios of Au and DAPT were respectively prepared as 1:0.125, 1:0.25, 1:0.5, 1:1, and 1:2, and were characterized by transmission electron microscopy, as shown in image 3 shown. , when the ratio of Au and DAPT is 1:0.125 to 1:0.25, some gold nanoparticles have larger particle size and are more likely to agglomerate. When the ratio of Au and DAPT is 1:0.5 to 1:2, the dispersion of gold nanoparticles is better, and the particle size is about 3nm.
[0041] Table 1 Surface molecular density of different gold nanoparticles
[0042]
[0043] According to the XPS results, the surface molecular density of gold nanoparticles was calculated, and the surface molecular density was the highest when the ratio of Au and DAPT was 1:1.
[0044] The above-mentioned gold nanoparticles with different ratios were tested toget...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com