Drug target of tumor related diseases and application thereof
A tumor-related and drug-based technology, applied in the direction of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve problems such as the unclear understanding of the mechanism of anti-tumor action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
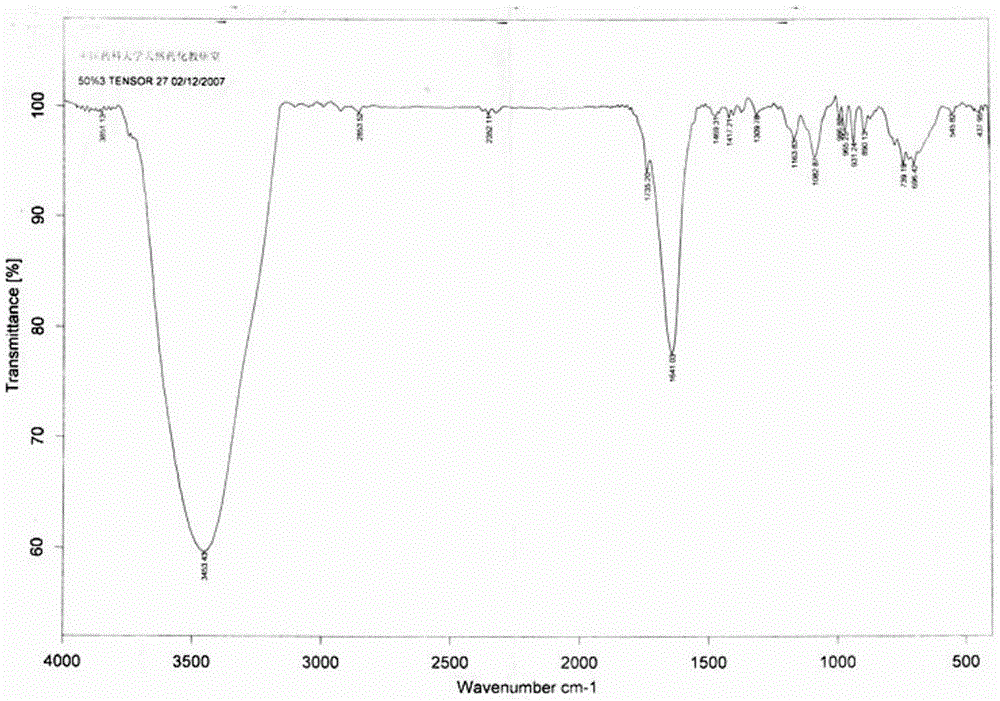
[0021] Example 1: Preparation of EAH Sepharose4B-PTL affinity column and investigation of column parameters
[0022]Take 10ml of EAH Sepharose4B and mix with 15mg of parthenol (PTL) lactone, and shake the reaction overnight at room temperature in ethanol solution containing 50% triethylamine. The reaction solution was centrifuged at 3000 rpm for 3 minutes, and the supernatant was reserved for investigating the coupling rate. The column material was washed with triple-distilled water to remove the reaction solvent, and stored in 30 ml of 20% ethanol solution for later use. Another small amount of reacted column material was washed with acetone to remove water, and infrared identification was carried out. Utilize high-performance liquid chromatography to detect the prepared affinity column material, and calculate coupling amount and coupling rate according to the following formula:
[0023] Coupling amount (mg / ml) = (the amount of added PTL - the amount of uncoupled PTL) / volu...
Embodiment 2
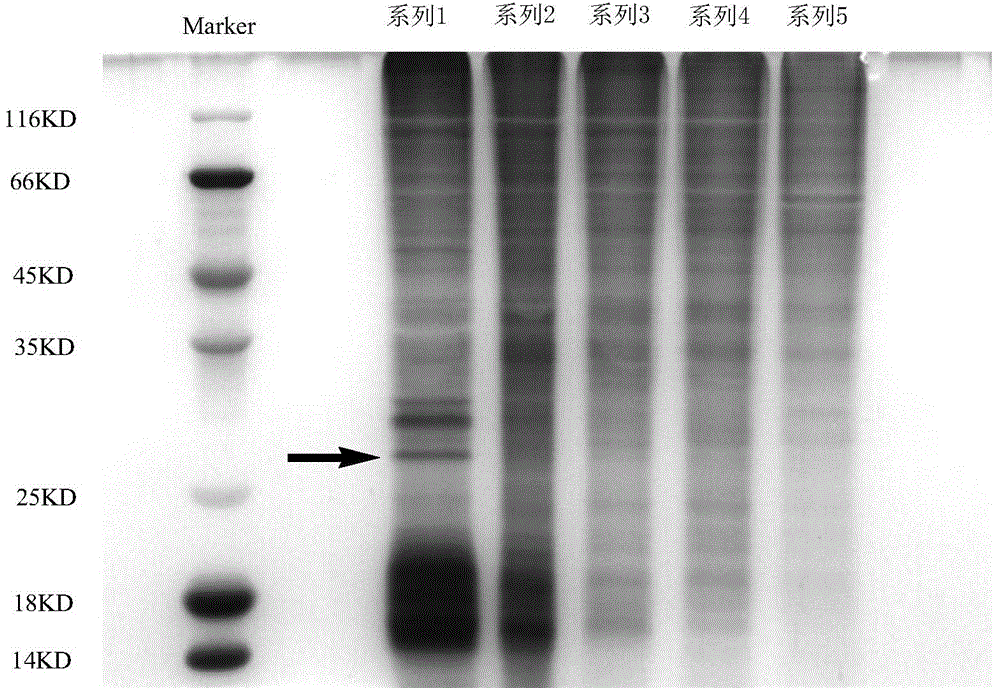
[0026] Example 2: Searching for proteins specifically binding to parthenolide by a series of affinity chromatography
[0027] In this experiment, human pancreatic cancer cells PANC-1 were cultured in DMEM high-glucose medium containing 10% fetal bovine serum. After the cells grow to an appropriate number, discard the medium and gently wash with PBS twice, add NP40 cell lysate and lyse on ice for 30 minutes to obtain the cell lysate, centrifuge at 12000rpm at 4°C for 15min, collect the supernatant, and use it for pro- and chromatography. Take 1ml PANC-1 cell lysate and add 100μl affinity column, mix evenly, shake in ice bath and centrifuge (3000rpm, 1min, 0-4℃), take the column sediment for identification, add 100μl supernatant again Affinity column material, mix well, and shake for 40min. According to this method, five consecutive affinity reactions were carried out, and all reactions were carried out in an ice bath. The column precipitates obtained from the 5 reactions wer...
Embodiment 3
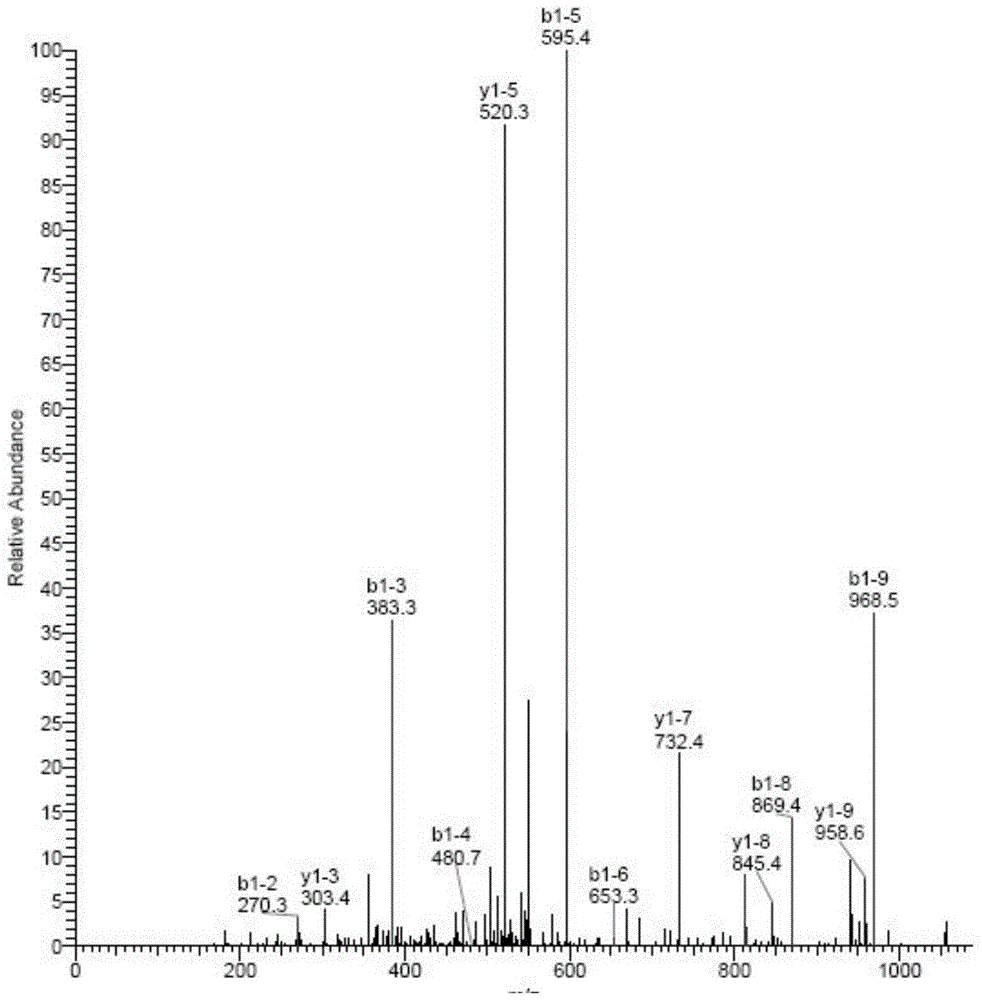
[0028] Example 3 A series of affinity chromatography to search for proteins specifically binding to parthenolide
[0029] Using the same method as in Example 2, cells from lung cancer (A549 cells, L78 cells, H460 cells), prostate cancer (PC-3 cells, DU145 cells), colorectal cancer (SW620 cells, LS513 cells), and bladder cancer were respectively cultured. Cancer (BIU-87 cells, 5637 cells, EJ cells), liver cancer (HepG2 cells, SMMC-7721 cells, Hep3b cells), nasopharyngeal carcinoma (C666-1 cells, CNE-1 cells), oral cancer (HB cells, KB Cell) ovarian cancer (A2780 cell, CAOV3 cell, SKOV-3 cell), cervical cancer (Hela cell), brain cancer (SF126 cell), neuroma (SH-SY5Y cell, M17 cell), lymphoma (DS-1 cell ), pancreatic cancer (BxPC-3 cells, SW1990 cells), gastric cancer (SGC7901 cells, MGC-803 cells), esophageal cancer (CaES-17 cells, Ec109 cells), laryngeal cancer (Hep-2 cells), tongue cancer (Tca8113 -P160 cells, TSCCA cells), breast cancer (MCF7 cells, A231 cells), skin cancer ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com