Recombinant porcine beta 2-adrenergic receptor protein and application thereof
A technology of adrenaline and recombinant protein, applied in the field of bioengineering, can solve the problems of poor activity and achieve the effect of ensuring activity and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 β 2 Adrenergic receptor gene Ss β 2 PCR amplification of AR
[0035] Beta of porcine (Sus scrofa) retrieved on NCBI 2 AR sequence (Genbank accession number AF000134), according to its two-terminal sequence, design a pair of primers, the 5' end of the upstream primer is introduced into the Nco Ⅰ restriction site, and the 5' end of the downstream primer is introduced into the Xhol Ⅰ restriction site, by RT-PCR β was amplified from the isolated and purified porcine hepatocyte total RNA 2 Adrenergic receptor gene Ss β 2 AR, its gene sequence is shown in SEQ ID No.2; its coded amino acid sequence is shown in SEQ ID No.1. The upstream primer (SEQ ID No.3) is: 5'-CAT GCCATGG CAGGGCAGCCCGGGAACCGC-3', the underlined sequence is the Nco I restriction site. The downstream primer (SEQ ID No.4) is 5'-CCG CTCGAG TCACAGCATGGAGTCATTTGTACTACAGTTCCTCC-3'. The underlined sequence is the Xhol I restriction site.
[0036] The reaction system is as follows:
[0037] ...
Embodiment 2
[0039] Example 2 β 2 Adrenergic receptor gene Ss β 2 Sequence analysis of AR and its encoded protein
[0040] beta 2 Adrenergic receptor gene Ss β 2 AR and published pig β 2 Compared with the AR gene (AF000134), the gene sequence identity is 99.68%, four base mutations occur, the amino acid sequence identity is 99.28%, three mutations occur, and β 2 Amino acids at the binding site of the agonist and the receptor are cloned correctly, which is suitable for the expression of active β in vitro 2 AR laid the groundwork.
Embodiment 3
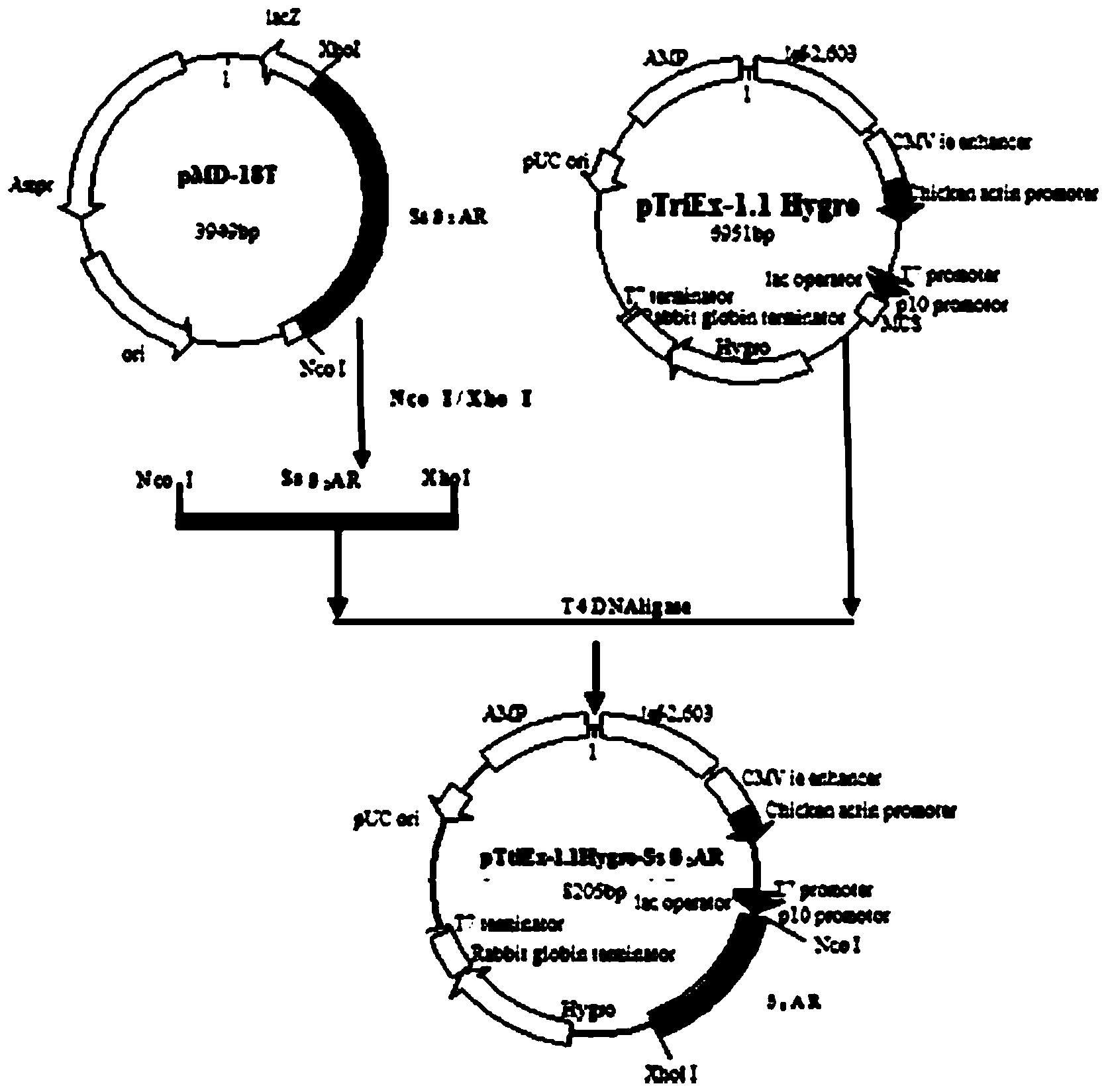
[0041] Embodiment 3PMD-18T-Ss β 2 Construction and Identification of AR Recombinant Cloning Plasmid
[0042] 1) PCR product purification
[0043] After the PCR product was subjected to 1% agarose gel electrophoresis, the target fragment was recovered by cutting the gel according to the operation steps of the DNA recovery and purification kit.
[0044] 2) Connection of target gene and cloning vector
[0045] The recovered product and the PMD-18T vector were double-digested with Nco Ⅰ and Xhol Ⅰ, respectively, and after 1% agarose gel electrophoresis, the double-digested product of the target gene and the large plasmid fragment were recovered. According to the molar ratio of 3:1, the T4 ligase Under the action, connect overnight at 4°C.
[0046] 3) Transformation and identification of recombinant cloning plasmids
[0047] The ligation product was transformed into DH5α competent cells and plated, and a single white colony was picked to extract the plasmid for PCR identificati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com