Preparation method for autologous-serum antigen-sensitized DC-CIK cells
A DC-CIK, autologous serum technology, applied in the fields of tumor immunotherapy and cell biology, can solve problems such as unfavorable promotion, high cost, serum pollution, etc., to avoid changes in antigen structure, low equipment requirements, and avoid resistance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The present invention is illustrated below with examples, but the present invention is not limited thereto. In the following examples, all the experimental methods that do not indicate the specific conditions are carried out in accordance with the conventional methods and the manufacturer's operating instructions.
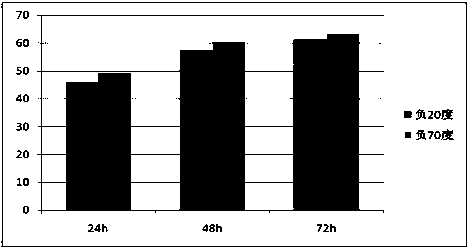
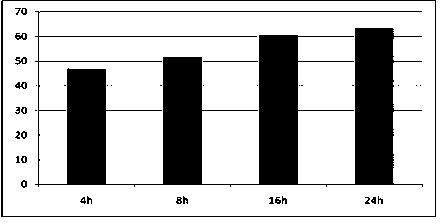
[0030] Routinely collect 50-100ml of peripheral blood from patients with advanced lung cancer, anticoagulate with 5-15IU / ml heparin sodium, rotate at 800g, centrifuge for 10 minutes, collect the upper plasma part (about 1 / 3 of the liquid layer), and add a final concentration of 0.1-1.0 mg / ml leupeptin inhibitor, mixed well, then stored in a sterile centrifuge tube, and placed in a freezer below -20°C for 24-48h (see figure 1 ), after taking it out, place it in an environment of 0-4°C for 10-16h (see figure 2 ); inactivated at 56°C for 30 minutes, and filtered through a 0.22 μm microporous membrane to obtain autologous serum tumor antigens.
[0031] Densit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com