Energetic salt based on 2, 4, 6-trinitro-1, 3-di(2', 4', 6'-trinitro-3'-hydroxyl styryl) benzene, synthetic method and application thereof
A technology of hydroxystyrene and synthetic methods, which is applied in the direction of preparation of amino compounds, chemical instruments and methods, aromatic nitration compositions, etc., can solve the problems of poor chemical and physical stability and low energy, and achieve improved heat resistance, Simple operation and high productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Mix and dissolve 2,4,6-trinitro-m-xylene (1.21g, 5.0mmol) and m-hydroxybenzaldehyde (1.28g, 10.5mmol) in 25mL of benzene and 25mL of dioxane, add pyridine (0.08g ) as a catalyst, and install a water separator at the same time. Slowly raise the temperature to reflux, and control the reflux rate to about 100 drops / min. The progress of the reaction was monitored by TLC and stopped when the reaction was almost complete (10 h). Part of the solvent (about 40mL) was removed, and the concentrated solution was separated by column chromatography. The developer ratio (n-hexane / ethyl acetate=5:1) was used to obtain 2.04g of a bright yellow solid with a yield of 91%.
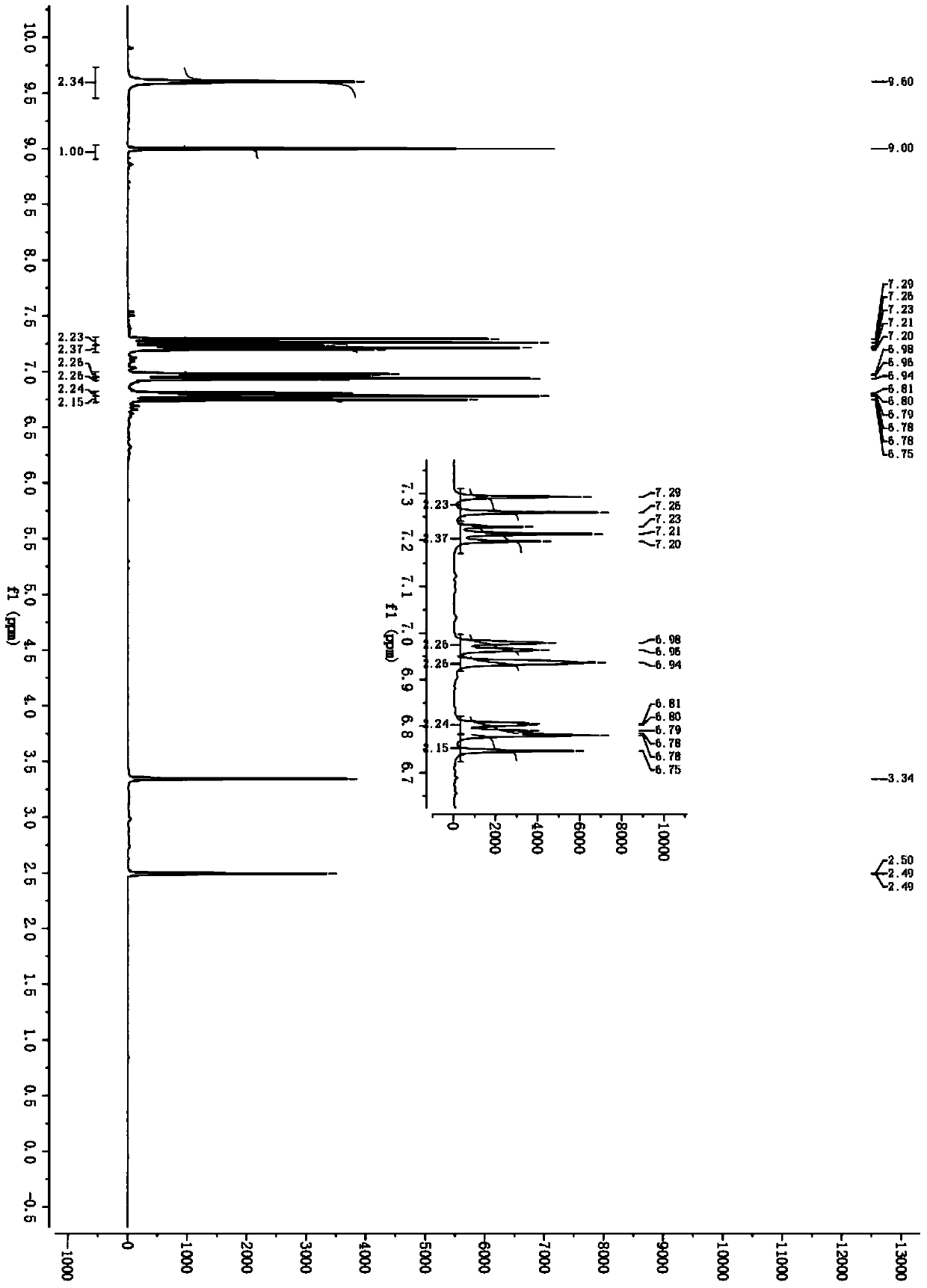
[0059] Bright yellow solid, melting point 194-196°C, IR (v, cm -1 ): 3400, 3095, 1635, 1609, 1586, 1514, 1395, 1323, 972; 1 H NMR (500MHz, DMSO-d 6 ), δ9.60(s, 2H), 9.00(s, 1H), 7.29(d, J=16.60Hz, 2H), 7.23(t, J=7.85Hz, 2H), 6.98(d, J=7.70Hz , 2H), 6.94(s, 2H), 6.81-6.78(dd, J 1 =8.10Hz,J 2 =1.75Hz, 2H), 6.78(d...
Embodiment 2
[0068] Mix and dissolve 2,4,6-trinitro-m-xylene (1.21g, 5.0mmol) and m-hydroxybenzaldehyde (1.28g, 10.5mmol) in 25mL of benzene and 25mL of dioxane, add morpholine (0.08 g) As a catalyst, install a water separator at the same time. Slowly raise the temperature to reflux, and control the reflux rate to about 100 drops / min. The progress of the reaction was monitored by TLC and stopped when the reaction was almost complete (10 h). Part of the solvent (approximately 40 mL) was removed, and the concentrated solution was separated by column chromatography. The developer ratio (n-hexane / ethyl acetate=5:1) was used to obtain 1.84 g of a bright yellow solid with a yield of 84%.
[0069] The following synthesis method follows the steps of Reference Example 1.
Embodiment 3
[0071] Mix and dissolve 2,4,6-trinitro-m-xylene (1.21g, 5.0mmol) and m-hydroxybenzaldehyde (1.28g, 10.5mmol) in 25mL of benzene and 25mL of dioxane, add morpholine (0.08 g) As a catalyst, install a water separator at the same time. Slowly raise the temperature to reflux, and control the reflux rate to about 100 drops / min. The progress of the reaction was monitored by TLC and stopped when the reaction was almost complete (10 h). Part of the solvent (about 40 mL) was removed, and the concentrated solution was separated by column chromatography. The developer ratio (n-hexane / ethyl acetate=5:1) was used to obtain 1.77 g of a bright yellow solid with a yield of 81%.
[0072] The following synthesis method follows the steps of Reference Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com