Preparation method of bromhexine hydrochloride
A technology of bromhexine hydrochloride and dibromobenzyl alcohol, which is applied in the novel preparation field, can solve the problems of low product yield, serious environmental pollution, complicated post-treatment, etc., and achieve improved yield and purity, good purity, and reduced The effect of the reaction step
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
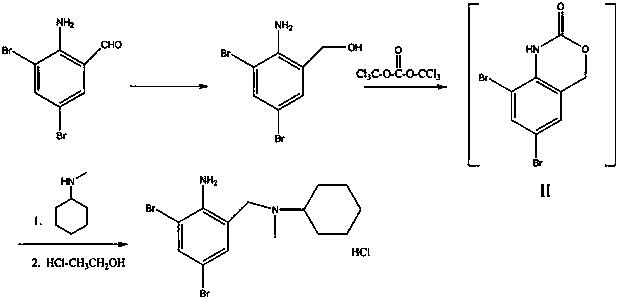
[0031] (1) 2-amino-3,5-dibromobenzyl alcohol
[0032] Add 2.5g of 2-amino-3,5-dibromobenzaldehyde into 25ml of solvent ethanol, and slowly add sodium borohydride solid (NaBH 4 ) 0.16g, keep the reaction at room temperature for 3 hours, after TLC detects that the reaction raw materials have basically disappeared, add water to dilute, use hydrochloric acid to acidify, adjust the pH to 5-6, continue to stir for 20 minutes, filter, wash with water, and vacuum dry to obtain Near white solid 2-amino-3,5-dibromobenzyl alcohol, melting point 152°C.
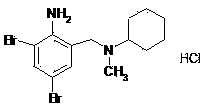
[0033] (2) 2-amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine hydrochloride (bromhexine hydrochloride)
[0034] Add 2g (about 7.12 mmol) of 2-amino-3,5-dibromobenzyl alcohol into 15ml of tetrahydrofuran (THF), and slowly add solid phosgene (triphosgene) in batches at 0-5°C under stirring conditions 0.72g, use sodium bicarbonate solution to absorb the HCl generated by the system, react at a temperature of 0-5°C for 2 hours, slowly add ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com