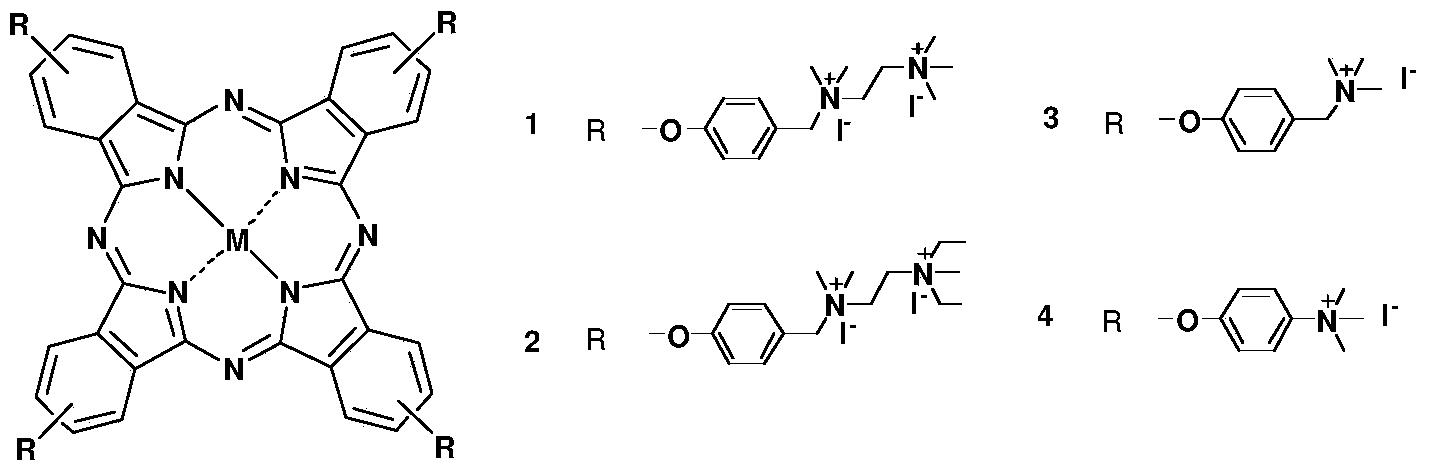
Cationic phthalocyanine as well as preparation and application thereof
A cationic and cationic technology, applied in the field of photosensitizers, can solve the problems of dissolution and aggregation of phthalocyanine photosensitizers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
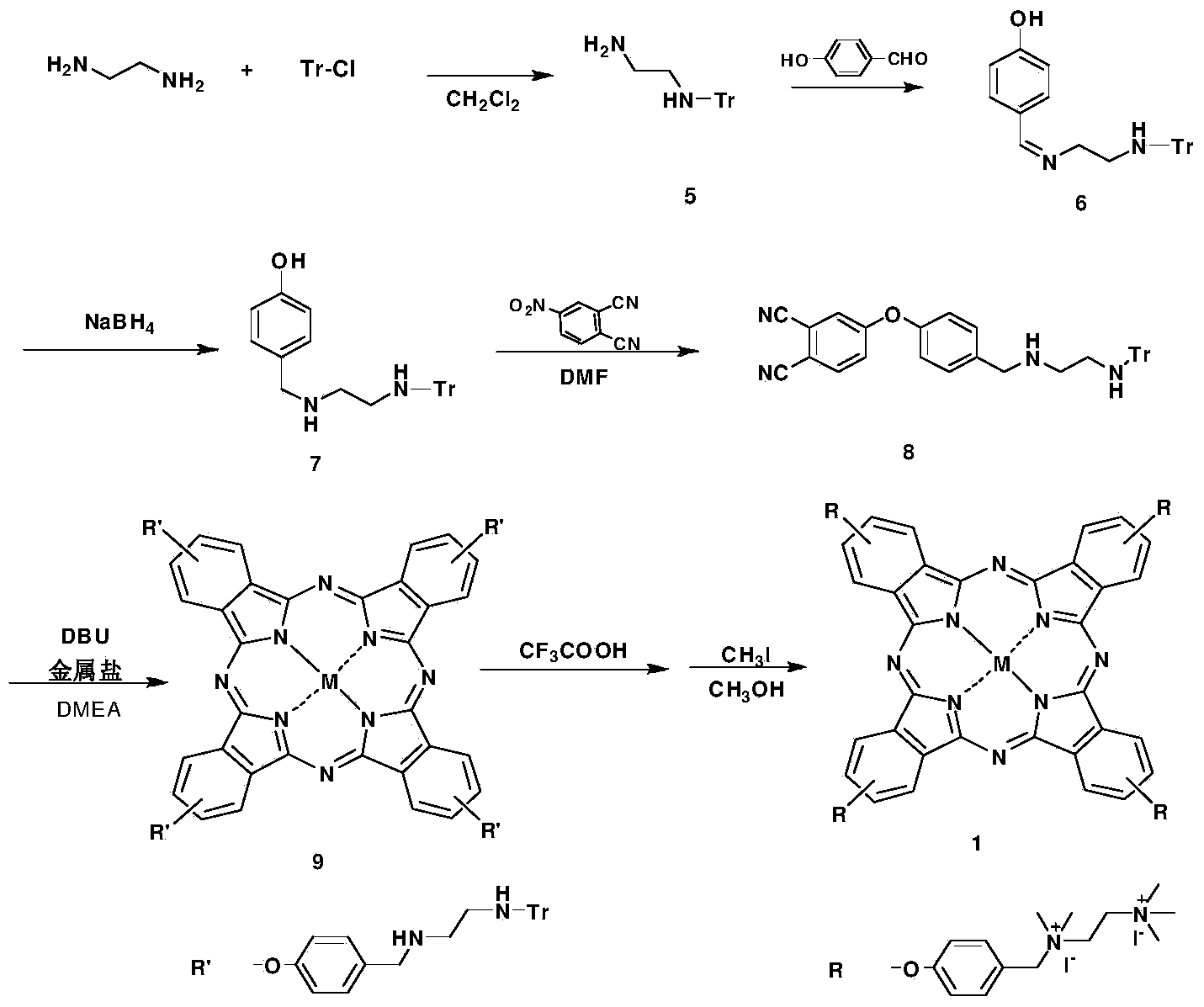
[0076] Cationic phthalocyanine 1 and preparation method thereof, represented by reaction formula as figure 2 as shown,
[0077] (1) Under the protection of argon, triphenylchloromethane is dissolved in chloroform, and slowly dripped into the chloroform solution of ethylenediamine and potassium carbonate at constant pressure. The molar ratio of triphenylchloromethane to ethylenediamine and potassium carbonate is 1 : 12: 2, about 2h of titration, reaction 4h, obtain intermediate 5;
[0078] (2) The molar ratio of intermediate 5 and 4-hydroxybenzaldehyde is 1:1.5, add methanol at room temperature, reflux for 3 hours, cool to room temperature, add 1 equivalent of sodium borohydride, continue stirring for 5 hours to obtain intermediate 7;
[0079] (3) Under the protection of argon, the molar ratio of intermediate 7 to 4-nitrophthalonitrile is 1:1.5, and N,N-dimethylformamide is used as a solvent, and the reaction is carried out at 60°C for 3 hours to obtain the intermediate 8;
...
Embodiment 2
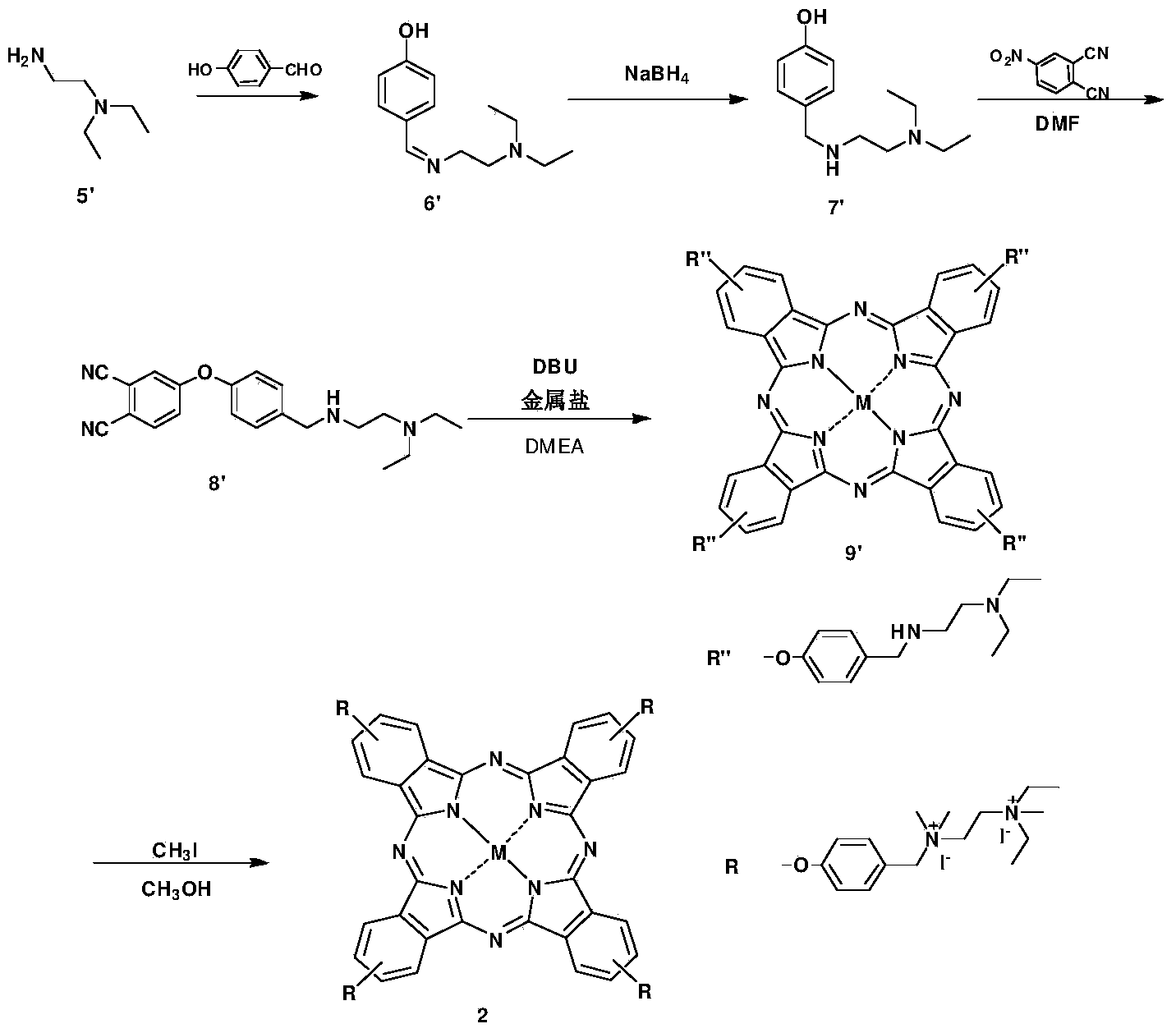
[0083] Cationic phthalocyanine 2 and preparation method thereof, represented by reaction formula as image 3 as shown,
[0084] (1) The molar ratio of diethylethylenediamine 5' to 4-hydroxybenzaldehyde is 1:1.5, add methanol at room temperature, reflux for 3 hours, cool to room temperature, add 1 equivalent of sodium borohydride, and continue stirring for 5 hours Obtain intermediate 7';
[0085] (2) Under the protection of argon, the molar ratio of intermediate 7' to 4-nitrophthalonitrile is 1:1.5, N,N-dimethylformamide is used as a solvent, and the reaction is carried out at 60°C for 3 hours to obtain the intermediate Body 8';
[0086] (3) Under argon protection, the intermediate 8', the metal salt ZnCl 2 Add 1,8-diazacyclo[5,4,0]undecene-7 into dimethylaminoethanol, react at 140°C for 12h to obtain phthalocyanine 9';
[0087] (4) Under an ice-water bath, phthalocyanine 9' was treated with methanol as a solvent, under the protection of argon, 40 equivalents of methyl iodi...
Embodiment 3
[0089] Cationic phthalocyanine 3, 4 and preparation method thereof, represented by reaction formula as Figure 4 as shown,
[0090] (1) Under argon protection, the molar ratio of p-hydroxyaniline or p-hydroxybenzylamine to 4-nitrophthalonitrile is 1:1.5, DMF is used as a solvent, and the reaction is carried out at 60° C. for 3 hours to obtain intermediate 10 (10');
[0091] (2) Under the protection of argon, the molar ratio of intermediate 10 (10') and paraformaldehyde is 1:6, the solvent is chloroform, and the reaction is refluxed for 4 hours; 4 equivalents of sodium borohydride are added in batches, and 10% is added after 2 hours of reaction The ammonia water is excessive, obtains intermediate 11 (11');
[0092] (3) Under argon protection, the intermediate 11 (11'), the metal salt ZnCl2 and DBU are added to MDEA in a molar ratio of 4:4:8, and reacted at 140°C for 12 hours to obtain phthalocyanines 12 and 12';
[0093] (4) Under the protection of argon, phthalocyanine 12 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com