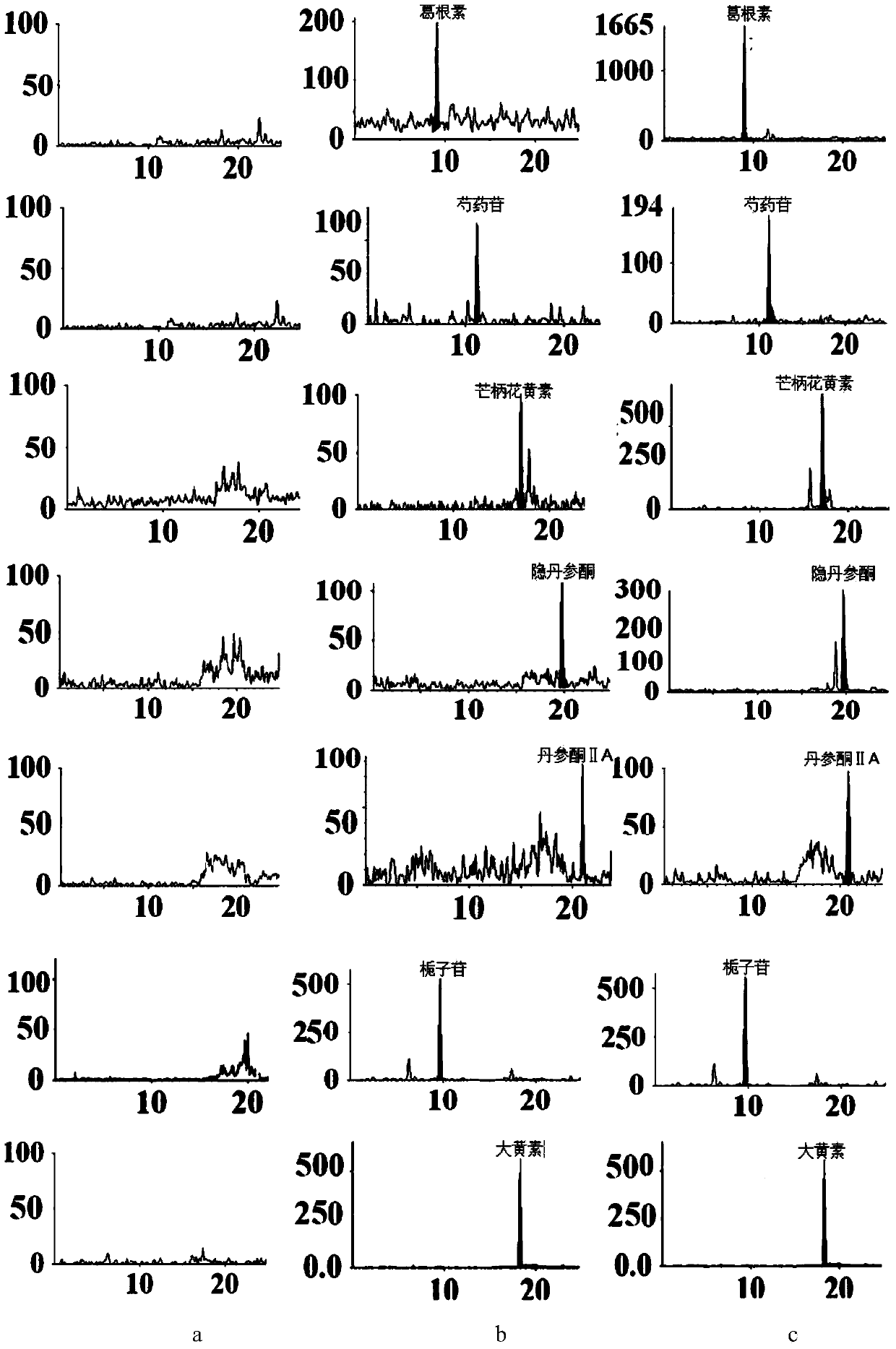
Method for simultaneously detecting main components of compound Danlou tablet in plasma
A technology of plasma and tanshinone, which is applied in the field of medicine, can solve problems that have not been reported, and achieve the effect of strong specificity and good sensitivity of the method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
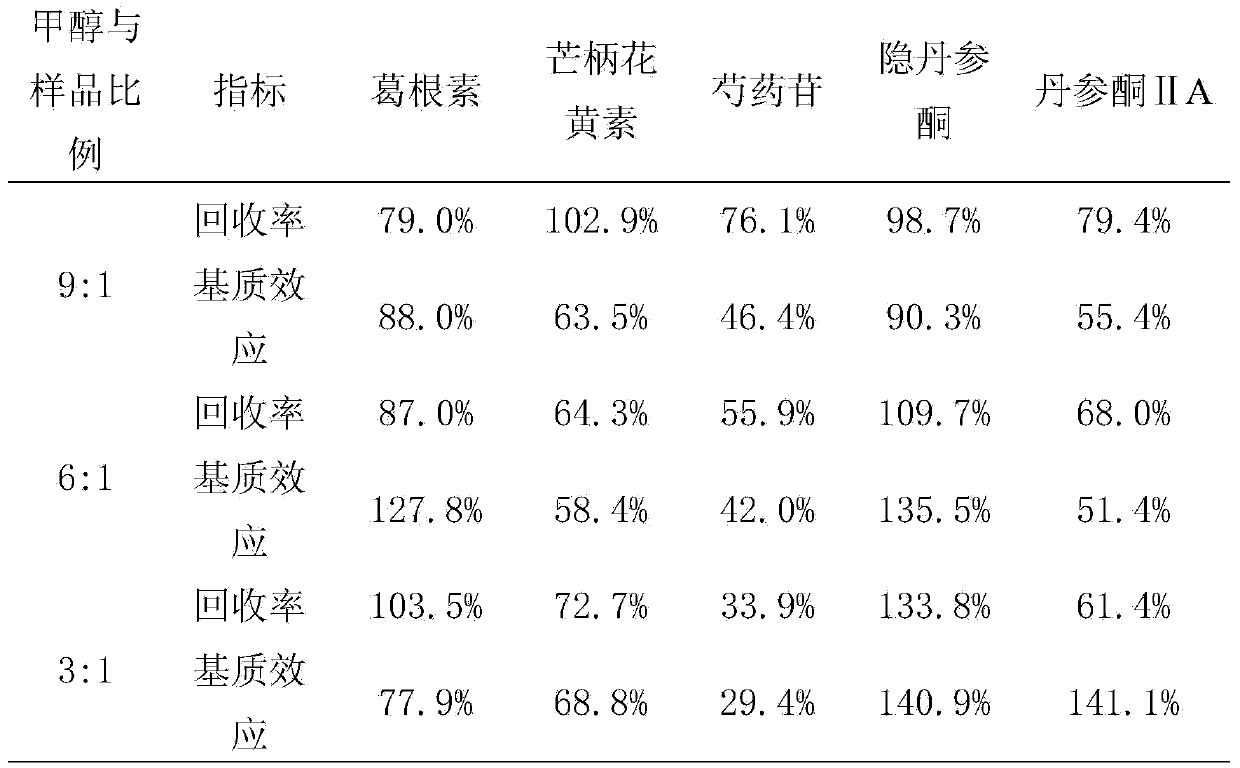
[0072] Example 1. Establishment of an analysis method for the main components of Fufang Danlou Tablets in plasma samples
[0073] LC-MS / MS conditions:
[0074] The column is Agilent Eclipse Plus C 18 Column (4.6mm×100mm, 1.8μm); guard column is Agilent C18 column (2.1×12.5mm, 5μm); mobile phase: A: acetonitrile, B: water (containing 0.1% formic acid by volume fraction); flow rate is 400μL / min; column temperature is 50°C; injection volume is 5 μL; time is 0-24min;
[0075] Gradient elution was adopted, and the elution program is shown in Table 2.
[0076] Table 2 Mobile phase gradient elution program
[0077]
[0078]
[0079] Mass spectrometry conditions: using API3200 TM LC / MS / MS liquid mass analysis system; Ion source, positive and negative ion fast switching analysis mode, MRM scanning mode;
[0080] Positive ion mode: curtain gas (CUR): 30psi; collision gas (CAD): 10psi; ion spray voltage (IS): 5500V; temperature (TEM): 450°C; airflow 1 (GS1): 30psi; airflow ...
Embodiment 2
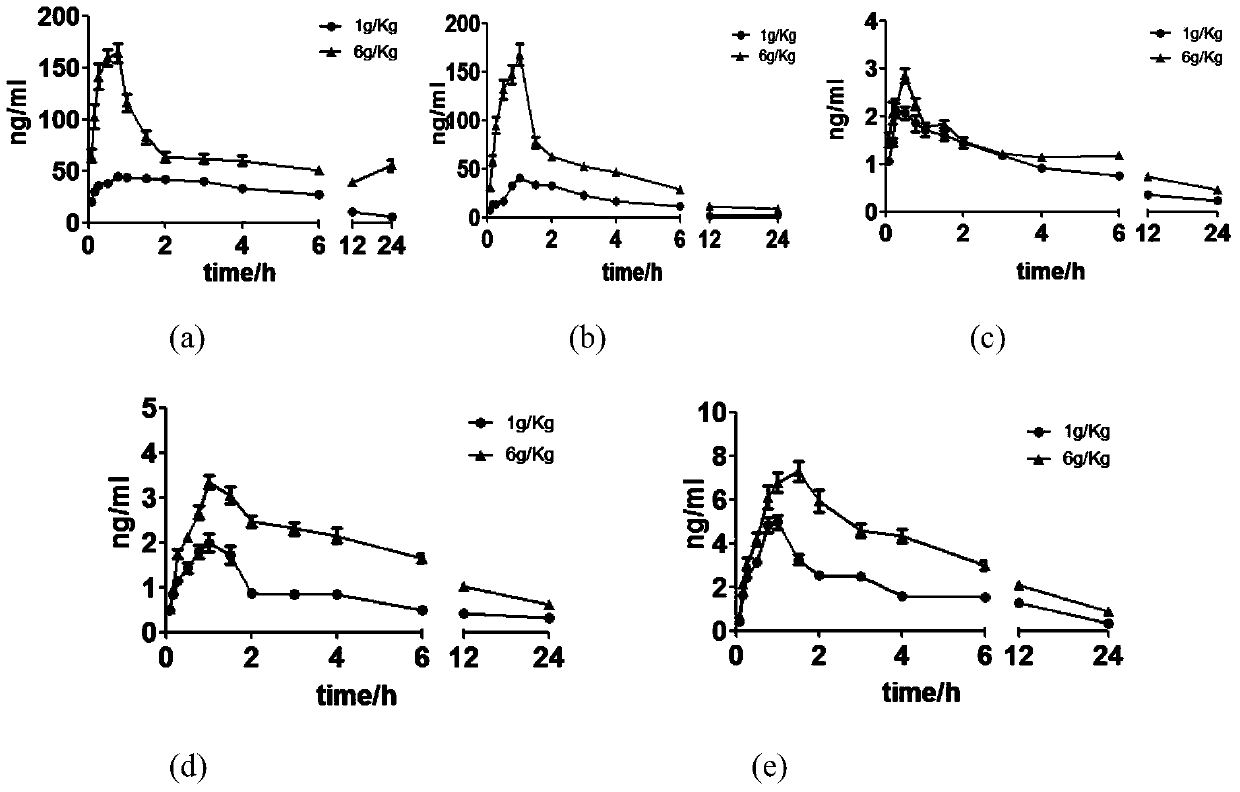
[0133] Embodiment 2, the pharmacokinetics of the main component of Compound Danlou Tablets after intragastric administration to rats
[0134] Contents of Five Compounds in Compound Danlou Tablets
[0135] LC-MS / MS was used to determine the contents of puerarin, paeoniflorin, formononetin, cryptotanshinone and tanshinone Ⅱ A in Fufangdanlan Tablets. The results showed that puerarin, paeoniflorin, formononetin and cryptotanshinone and tanshinone Ⅱ A content were 70.95mg / g, 21.07mg / g, 0.28mg / g, 1.33mg / g and 1.26mg / g.
[0136] Collection of plasma samples
[0137] The male SD rats under test were pre-adapted for one week, fasted without food and water for 12 hours the night before the test, and on the second day, the experimental animals were randomly divided into two groups, with 8 rats in each group, and 1 g·kg -1 and 6g·kg -1 Dose administered by intragastric administration, blood was collected from the fundus venous plexus at 0.083, 0.167, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| collision gas | aaaaa | aaaaa |
| collision gas | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com