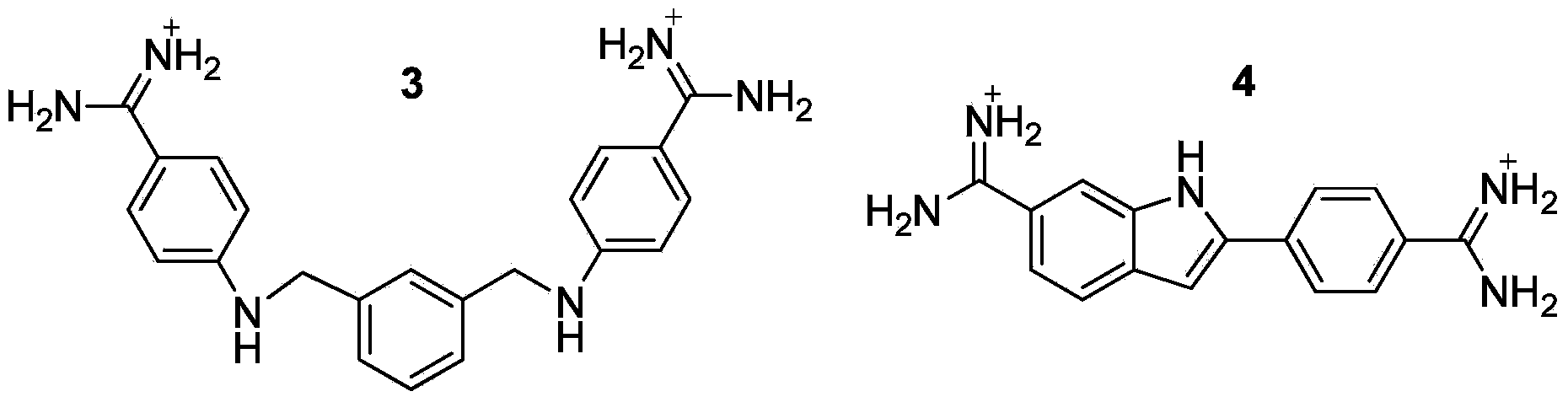
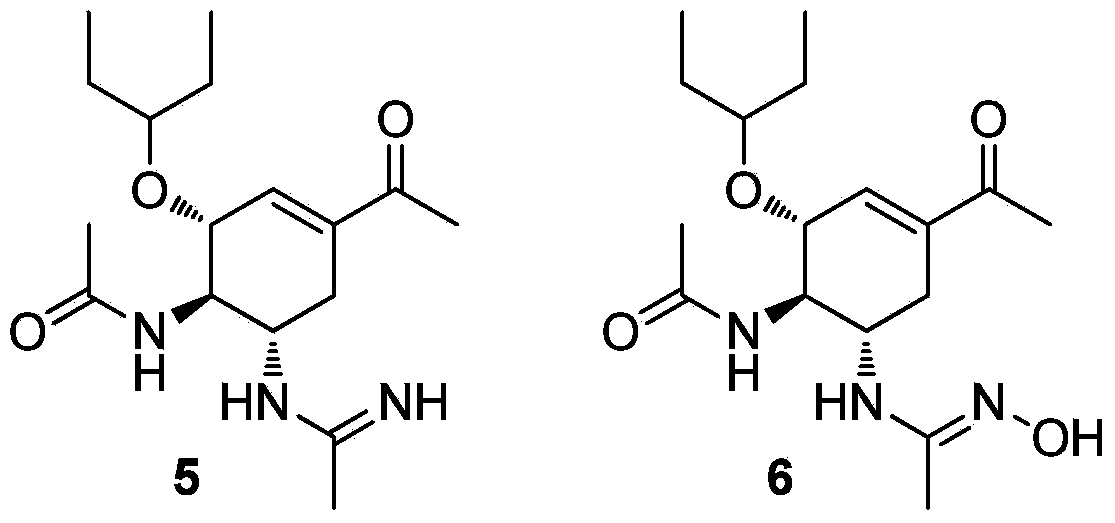
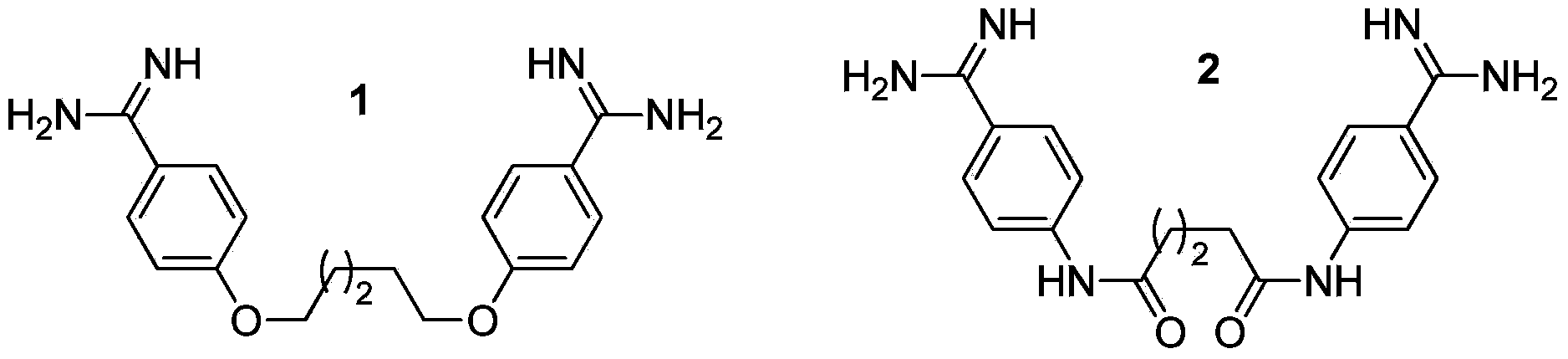Multi-substituted amidine compound as well as preparation method and application thereof
A polysubstituted, cyclic amidine technology, applied in the preparation of sulfonic acid amides, organic chemistry, drug combination, etc., can solve problems such as lack of structural diversity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Under nitrogen protection, 1.0 mmol of allylamine alkyne 9a (R=CH 2 CHCHCO 2 Et, R 1 = H, R 2 =H, n=1), 1.1 mmoles of p-toluenesulfonyl azide, and 1.0 moles of diisopropylethylamine were mixed in 5 ml of toluene, and 0.01 moles of 2-thiazole formic acid copper was added at -30 degrees, Stir and mix evenly. After 5 hours, add 2 ml of saturated ammonium chloride solution, continue stirring for 5 hours, wash with 5 ml of water and 5 ml of saturated saline successively, separate the organic phase and dry it with anhydrous sodium sulfate, filter and remove the organic solvent , add 1 g of 200-mesh silica gel and 6 ml of dichloromethane and mix well, carefully distill off the solvent, put the remaining silica gel on a silica gel column, elute with ethyl acetate / petroleum ether, collect the fractions, and obtain 43% cyclic amidine 7a.
[0045] 7a: Yellow oil, 43%; 1 H NMR (400MHz, CDCl 3 )δ7 . 77(d, J=8.3Hz, 2H), 7.18(d, J=8.0Hz, 2H), 6.01-5.92(m, 1H), 5.60(ddt, J=16...
Embodiment 2
[0047] Under nitrogen protection, 1.0 mmoles of alkyne 9b Mix 1.1 mmol of p-toluenesulfonyl azide and 10.0 moles of diisopropylethylamine in 50 ml of toluene, add 0.10 moles of copper 2-thiazole formate at 60 degrees Celsius, stir and mix evenly, after 10 minutes, add 2 milliliters of saturated ammonium chloride solution, continued to stir for 5 hours, washed successively with 5 milliliters of water and 5 milliliters of saturated saline, separated the organic phase and dried it with anhydrous sodium sulfate, filtered and removed the organic solvent, added 1 gram of 200 mesh silica gel and 6 Mix a milliliter of dichloromethane evenly, carefully distill off the solvent, put the remaining silica gel on a silica gel column, elute with ethyl acetate / petroleum ether, and collect fractions to obtain 96% cyclic amidine 7b.
[0048] 7b: 94%; 1 h NMR (400MHz, CDCl 3 )δ7 . 79(d, J=8 . 2Hz, 2H), 7.20(d, J=8.0Hz, 2H), 7.07(d, J=8.6Hz, 2H), 6.77(d, J=8.7Hz, 2H), 5.89-5.78(m, 1H), ...
Embodiment 3
[0051]
[0052] 7c: 88%; 1 H NMR (500MHz, CDCl 3 )δ7.76(d, J=8.3Hz, 2H), 7.26-7.25(m, 3H), 7.18(d, J=8.1Hz, 2H), 7.14-7.12(m, 2H), 5.85(dddd, J =16.8,10.1,9.1,5.2Hz,1H),5.13-5.09(m,2H),4.75(d,J=14.5Hz,1H),4.53(d,J=14.4Hz,1H),3.90-3.87( m,1H),3.37-3.25(m,2H),3.04-3.01(m,1H),2.37(s,3H),2.31-2.24(m,1H),1.89-1.86(m,2H),1.75- 1.70(m,2H); 13 C NMR (125MHz, CDCl 3 )δ168.8, 141.8, 141.6, 135.9, 135.5, 129.0, 128.7, 128.0, 127.7, 126.0, 117.70, 53.5, 48.2, 36.8, 36.4, 21.7, 21.4, 17.3; HRMS (ESI) m / z theoretical value C 22 h 26 N 2 o 2 SNa + [M+Na] + 405.1607, measured value 405.1601.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com