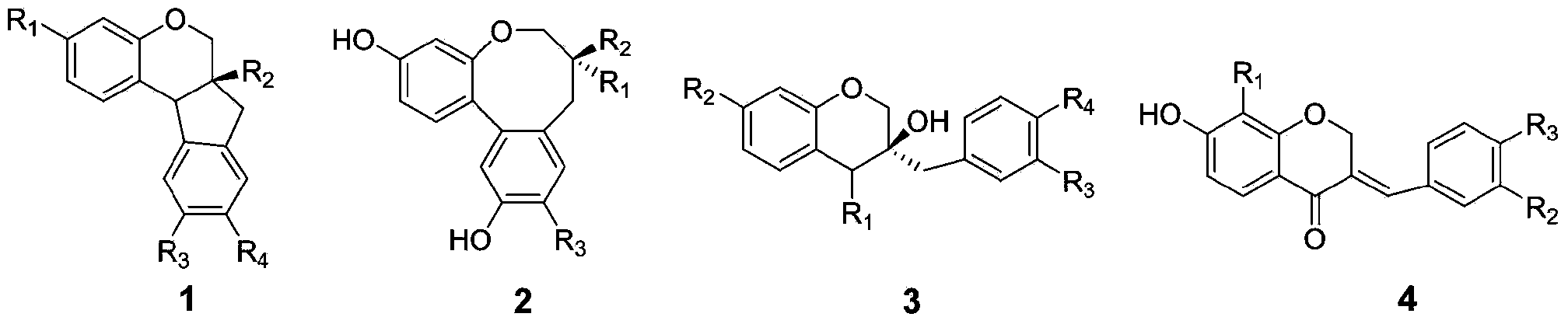
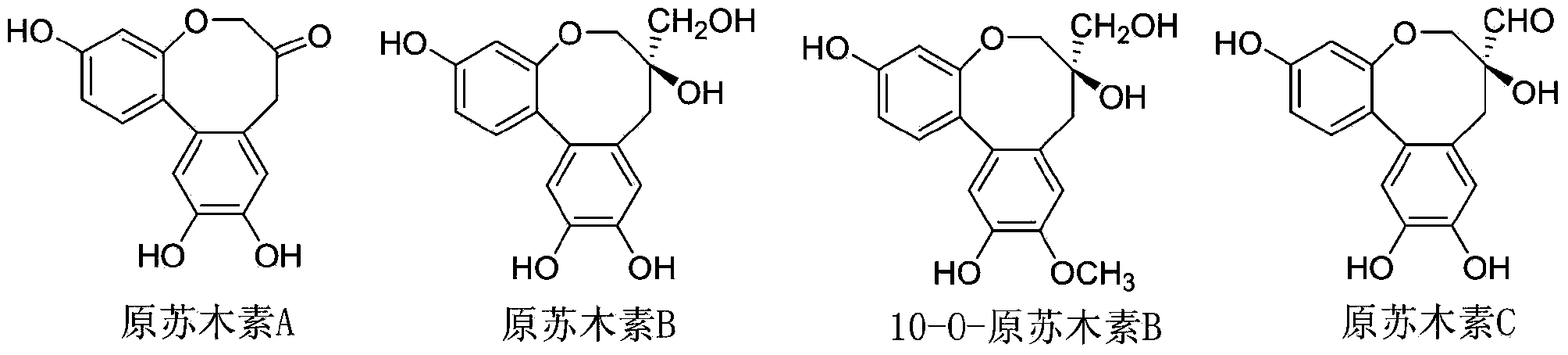
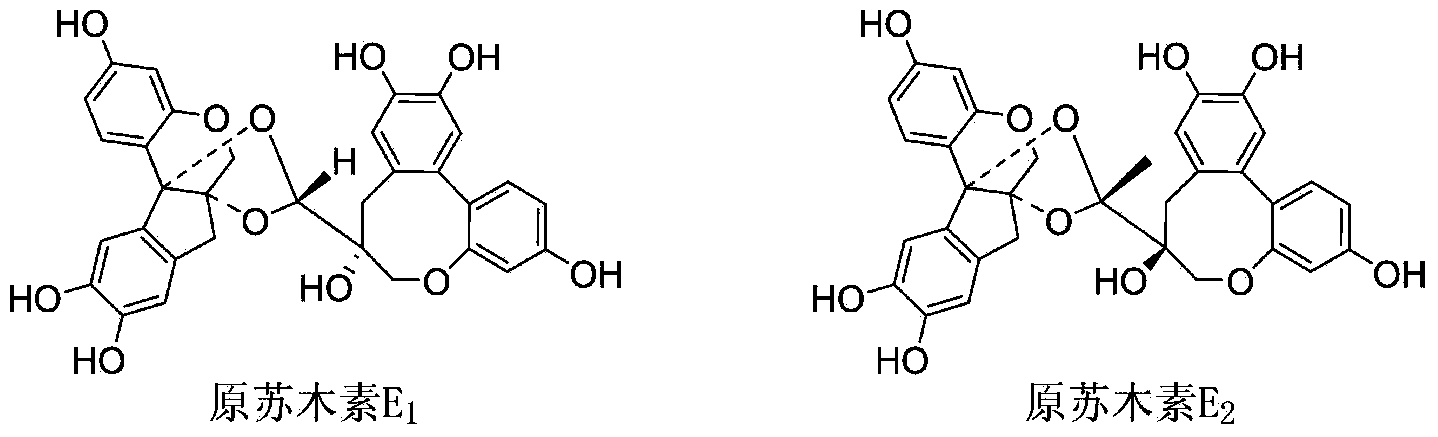
Biochemical total synthetic method for protosappanin A and derivatives thereof
A synthesis method and hematoxylin technology, applied in the direction of organic chemistry, etc., can solve the problems such as the specific mechanism needs to be further studied, the scavenging of free radicals is weak, etc., and the effect of good industrial production prospects, easy operation and high yield can be achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0059] Example 13,10,11-trimethoxy-6H-dibenzo[b,d]oxetane-7(8H)-one
[0060]
[0061] 3,9,10-Trimethoxydibenzo[d,f]oxepan-3-one (400mg, 1.33mmol), zinc iodide (85mg, 0.27mmol), dichloromethane (5mL) were added Add cyanotrimethylsilane (450 mmL, 3.33 mmol) into the reaction flask, and stop the reaction at room temperature for 12 hours. Add 6% NaHCO to the reaction solution 3 The solution (10 mL) was reacted at room temperature for 30 minutes, the aqueous phase was separated, the organic phase was dried over anhydrous sodium sulfate for 2 hours, filtered, and dichloromethane was spun off to obtain a seven-membered cyclocyanosilyl ether compound (430 mg, 1.30 mmol). The obtained seven-membered ring cyanide was dissolved in 30 mL of anhydrous ether, lithium aluminum hydride (126 mg, 3.33 mmol) was added, and the reaction was carried out at room temperature for 20 hours under airtight conditions. After the reaction was completed, the reaction was quenched by adding a small amo...
Embodiment 23
[0069] Example 23-Methoxy-6H-dibenzo[b,d]oxetane-7(8H)-one
[0070]
[0071] 3-Methoxydibenzo[d,f]oxepan-3-one (400mg, 1.66mmol), zinc iodide (106mg, 0.33mmol), dichloromethane (5mL) were added to the reaction flask , add cyanotrimethylsilane (560mmL, 4.16mmol), and stop the reaction at room temperature for 12 hours. Add 6% NaHCO to the reaction solution 3 The solution (10 mL) was stirred at room temperature for 30 minutes, the aqueous phase was separated, the organic phase was dried over anhydrous sodium sulfate for 2 hours, filtered, and dichloromethane was spun off to obtain a seven-membered cyclocyanosilyl ether compound (436 mg, 1.60 mmol). The obtained seven-membered ring cyanide was dissolved in 30 mL of anhydrous ether, lithium aluminum hydride (158 mg, 4.16 mmol) was added, and the mixture was reacted at room temperature for 20 hours under airtight conditions. After the reaction was completed, the reaction was quenched by adding a small amount of water in an ice ...
Embodiment 310
[0079] Example 310,11-trimethoxy-6H-dibenzo[b,d]oxetane-7(8H)-one
[0080]
[0081] 9,10-Dimethoxydibenzo[d,f]oxepan-3-one (400mg, 1.48mmol), zinc iodide (95mg, 0.29mmol), dichloromethane (5mL) were added to In the reaction bottle, cyanotrimethylsilane (500 mmL, 3.70 mmol) was added, and the reaction at room temperature was stopped for 12 hours. Add 6% NaHCO to the reaction solution 3 The solution (10 mL) was stirred at room temperature for 30 minutes, the aqueous phase was separated, the organic phase was dried over anhydrous sodium sulfate for 2 hours, filtered, and dichloromethane was spun off to obtain a seven-membered cyclocyanosilyl ether compound (435 mg, 1.44 mmol). The obtained seven-membered ring cyanide was dissolved in 30 mL of anhydrous ether, lithium aluminum hydride (158 mg, 4.16 mmol) was added, and the mixture was reacted at room temperature for 20 hours under airtight conditions. After the reaction was completed, a small amount of water was added to quen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com