Synthesis method of high-sensitivity carbendazol complete antigen
A technology of complete antigen and synthesis method, applied in the field of synthesis of complete antigen of carbendazim, to achieve the effect of high sensitivity and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
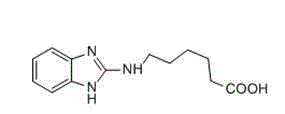
[0034] The preparation of embodiment 1 carbendazim hapten H1
[0035] (1) Synthesis of precursor 261: Aminocaproic acid methyl ester hydrochloride (obtained by refluxing aminocaproic acid and thionyl chloride in methanol) and equimolar amounts of 2-chlorobenzimidazole were mixed in diiso Microwave reaction in propylethylamine at 150°C overnight for 13h yielded about 10% of the product, and the intermediate was obtained through preparation and purification.
[0036] (2) Synthesis of hapten H1: Dissolve the above intermediate in tetrahydrofuran (THF) , Stir the reaction overnight at room temperature, remove THF by rotary evaporation, adjust the pH to 6~7 with 1mol / L HCl solution, and purify the product by preparative chromatography.
Embodiment 2
[0037] Embodiment 2 Carbendazim hapten H1 prepares complete antigen
[0038] 1) Activation of hapten H1: Take 35 mg of carbendazim hapten H1, add 2 mL of DMF to dissolve, then add NHS and EDC respectively (the molar ratio of hapten, NHS, and EDC is 1: 1.5: 2), and stir at room temperature for 12 hours .
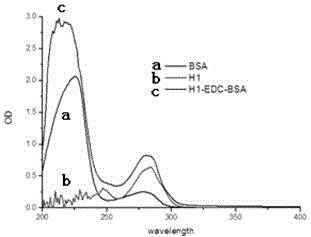
[0039] 2) Preparation of protein solution: Take 160mg of bovine serum albumin (the molar ratio of hapten H1 to bovine serum albumin is 60:1), add 10mL of 0.1M pH9.6 carbonate buffer.
[0040] 3) Reaction: slowly drop the activated hapten H1 solution prepared in step 1) into the protein solution prepared in step 2), and react at room temperature for 8 hours. Dialyze with PBS buffer solution for 3 days, during which the water was changed 6 times, and the complete antigen of carbendazim was obtained.
Embodiment 3
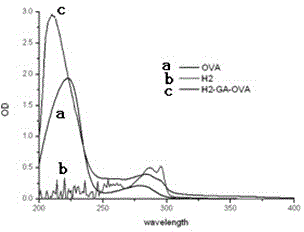
[0041] Implementation example 3, carbendazim hapten H2 preparation coating original
[0042] The structural formula of H2 is:
[0043]
[0044] Take 20 mg of carbendazim hapten H2, add 1 mL of DMF to dissolve, and pre-cool at 0°C for 30 min. At 0°C, add glutaraldehyde (the molar ratio of hapten to glutaraldehyde is 1:1.2), and react at 0°C for 20 minutes. Take 120mg of chicken ovalbumin (the molar ratio of hapten to chicken ovalbumin is 30:1), add 10mL of 0.1M pH9.6 carbonate buffer, and pre-cool at 0°C for 30 min. At 0°C, the activated hapten solution was slowly added dropwise to the protein solution, and reacted at 0°C for 4 hours. Dialyze with PBS buffer solution for 3 days, during which the water was changed 6 times to obtain the carbendazim-coated original.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com