Method for determining chemical oxygen demand of high-chlorine waste water
A chemical oxygen demand and water sample technology, which is applied in the direction of material analysis by observing the influence of chemical indicators, and analysis by making materials undergo chemical reactions, can solve the difficulties of mercury sulfate recovery, personal injury, secondary environment, etc. Pollution and other problems, to achieve the effect of ensuring catalytic oxidation ability, preventing secondary pollution, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
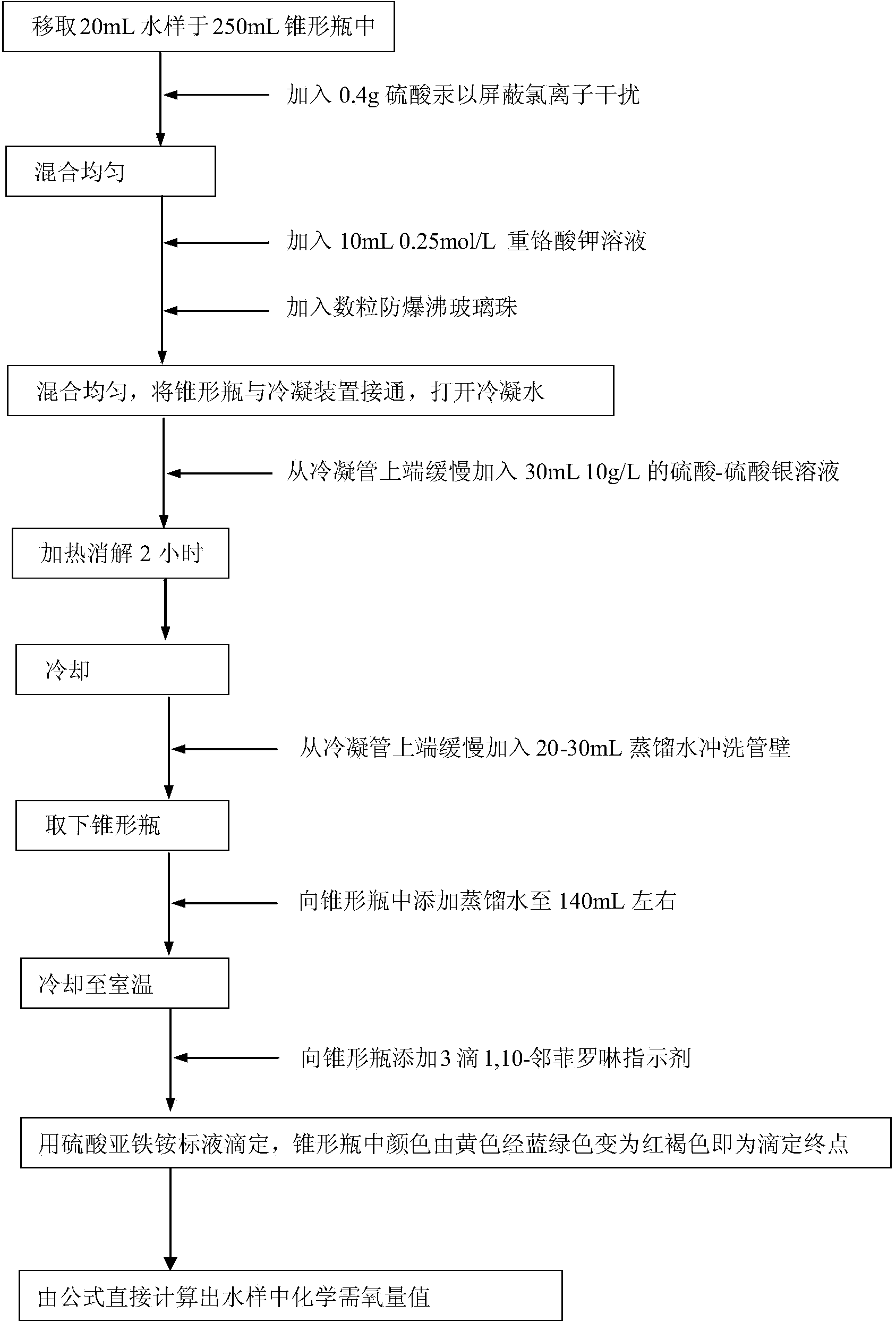
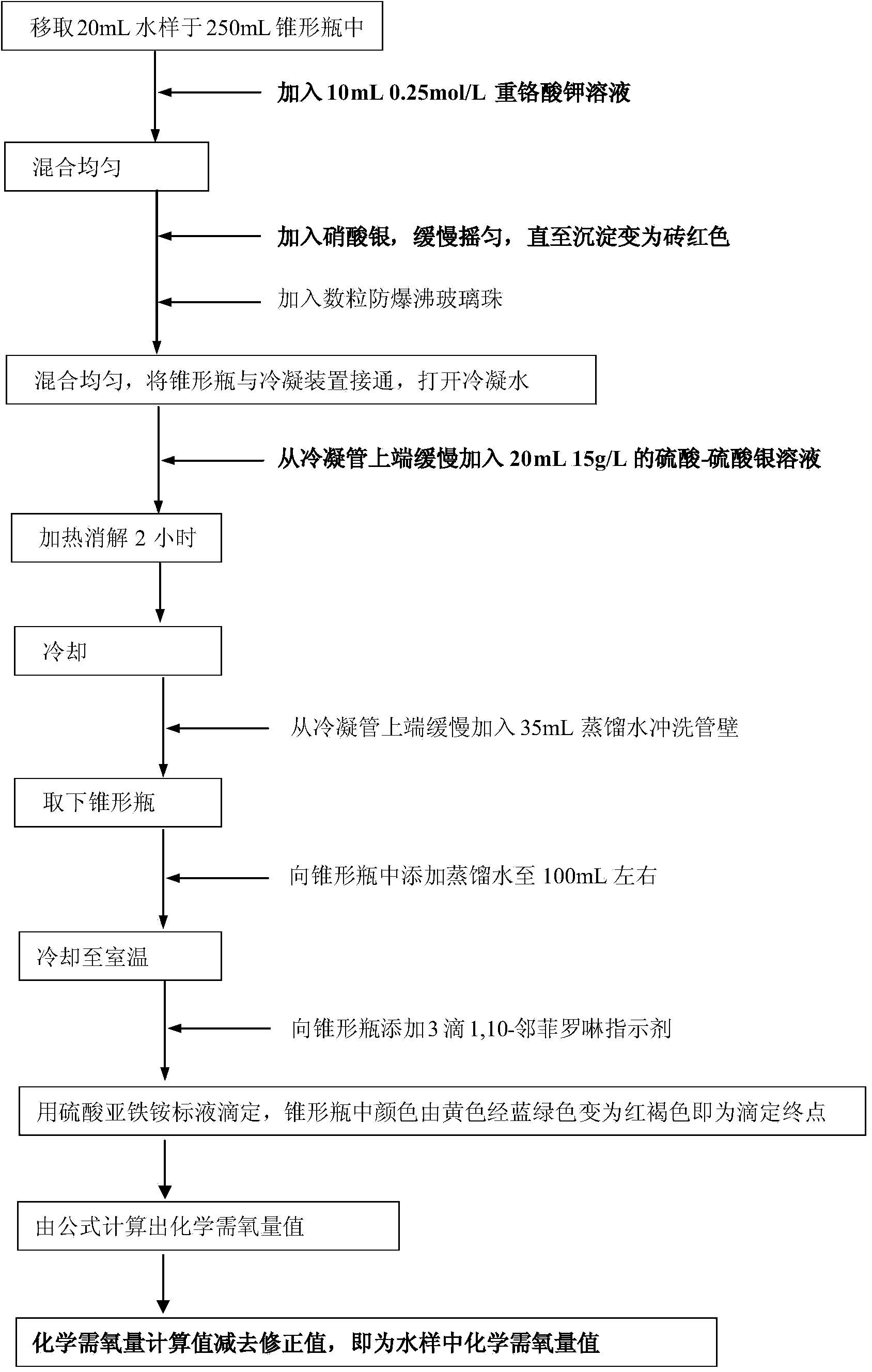
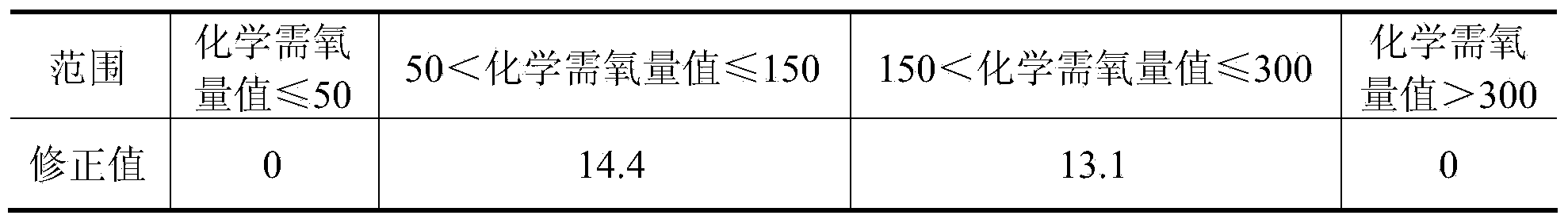
[0036]Use sodium chloride and potassium hydrogen phthalate to prepare a series of standard solutions with a certain concentration of chloride ion and chemical oxygen demand value. The chloride ion is set to 3000, 5000, 8000, 12000, 15000, 18000, 20000mg / L7 gradients, chemical Oxygen demand is set to 6 gradients of 50, 100, 200, 300, 500, 700mg / L, a total of 42 samples. According to the assay method provided by the invention ((1) add potassium dichromate solution in the water sample of packing into Erlenmeyer flask, then mix homogeneously; (2) adopt silver nitrate to add the water sample of Erlenmeyer flask as chloride ion shielding agent In the process, after the chlorine ions and silver nitrate in the water sample completely form a silver chloride precipitate, the excess silver nitrate combines with potassium dichromate to form a brick red silver chromate precipitate, and the brick red precipitate in the Erlenmeyer flask is the end point of silver nitrate addition. (3) add th...
Embodiment 2
[0041] Use sodium chloride and potassium hydrogen phthalate to prepare a series of standard solutions with a certain concentration of chloride ion and chemical oxygen demand value. The chloride ion is set to 10000, 20000mg / L2 gradients, and the chemical oxygen demand is set to 100, 300mg / L2. Gradient, a total of 4 samples, according to the method in Example 1, each sample was repeatedly measured 6 times, and the relative standard deviation was calculated. The results are shown in Table 2.
[0042] Table 3 Precision test results Unit: mg / L
[0043]
[0044] It can be seen from Table 3 that under the conditions of this example, the relative standard deviation of multiple determinations of samples by the method of the present invention is between 1.9% and 3.6%, and the precision is good.
Embodiment 3
[0046] Adopt the method of the present invention to measure the chemical oxygen demand value of 4 different samples respectively (domestic sewage sample 1, domestic sewage sample 2, production wastewater sample 1, production wastewater sample 2), then add standard to 4 samples respectively (standard substance Potassium hydrogen phthalate), and adopt the method in embodiment 1 to measure the COD value after standard addition, calculate standard addition recovery, experimental result is shown in Table 4.
[0047] Table 4 The experimental results of the standard recovery rate
[0048]
[0049] As can be seen from Table 3, under the conditions of this example, the standard addition recovery rate of the method of the present invention is between 94.0%-109.8%, and the standard addition recovery rate is good.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com