A kind of human insulin monoclonal antibody cross-linked magnetic particles and its preparation method and human insulin detection kit including the same
A monoclonal antibody and detection kit technology, applied in the field of in vitro diagnostic medical testing, can solve the problems of poor repeatability of insulin kits, unfavorable promotion of insulin detection, poor clinical application prospects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] Correspondingly, the present invention also provides a method for preparing the above-mentioned human insulin monoclonal antibody cross-linked magnetic particles, the method comprising the following steps:
[0053] a) treating the human insulin monoclonal antibody with a nonionic surfactant; and
[0054] b) cross-linking the human insulin monoclonal antibody treated in step a) with magnetic particles.
[0055] The human insulin monoclonal antibody cross-linked magnetic particles of the present invention can be used to detect human insulin or prepare a human insulin detection kit, thereby significantly improving the sensitivity of human insulin detection.
[0056] The term "nonionic surfactant" as used herein has the meaning commonly understood by those of ordinary skill in the art, ie, a surfactant that does not generate ions in aqueous solution. Nonionic surfactants do not dissociate when dissolved in water, and the lipophilic groups in their molecules are roughly the...
Embodiment 1
[0124] Example 1. Pretreatment of human insulin monoclonal antibody
[0125] 1) Dilute human insulin antibody (BM1, Roche) with 10mM pH7.40 PBS solution to 2mg / ml;
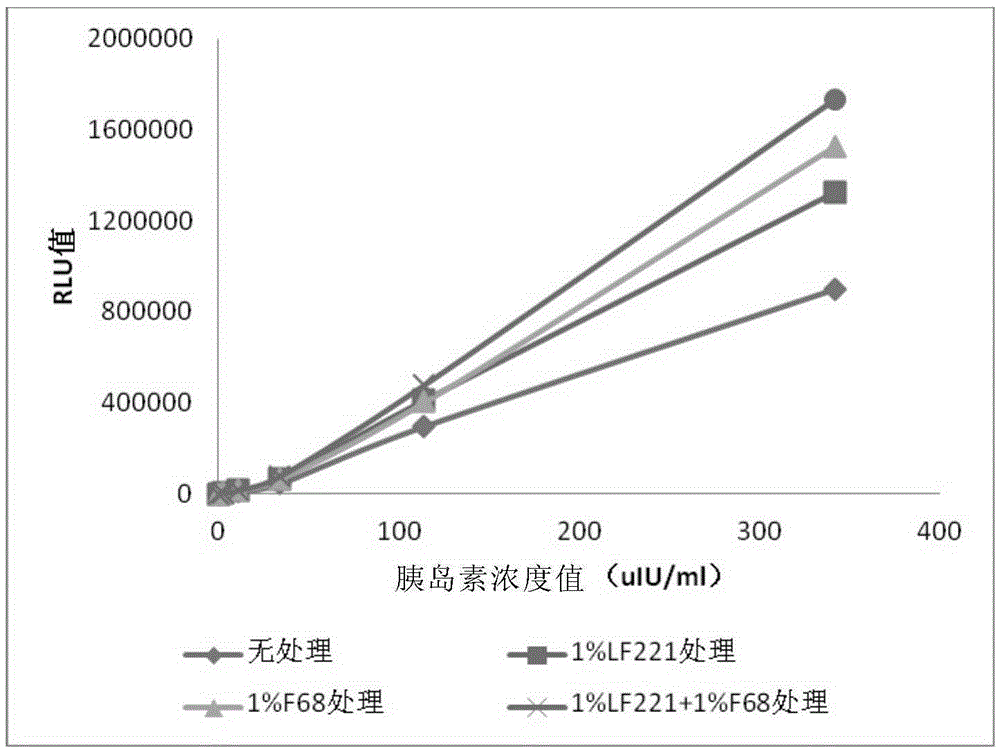
[0126] 2) the nonionic surfactant LF221 and F68, respectively diluted to 1% (mass percentage concentration) with purified water;
[0127] 3) Mix human insulin antibody with 1% LF221 or 1% F68 is mixed according to the volume ratio of 9:1; or human insulin antibody and 1% LF221, 1% For F68, mix according to the volume ratio of 8:1:1, and mix for 30 minutes at 2-8°C;
[0128] 4) Put the treated insulin antibody in 10mMpH7.40PBS solution, and dialyze at 37°C for 4-8 hours;
[0129] 5) Take out the dialysate and keep the insulin antibody at 2-8°C for use.
Embodiment 2
[0130] Example 2. Preparation of Human Insulin Monoclonal Antibody Crosslinked Magnetic Particles
[0131] 1) Take 10 mg of 1.0 μm magnetic particles whose active functional groups are carboxyl groups, and wash them twice with 50 mM MES buffer at pH 6.0;
[0132] 2) Remove the supernatant after magnetic suction, add 0.5mL of 50mM pH6.0 MES buffer and mix well, then add 0.5mL of 25mg / mL carbodiimide (EDC) solution and mix well;
[0133] 3) react at room temperature for 30 minutes;
[0134] 4) Remove the supernatant after magnetic suction, and wash twice with 50mM pH6.0 MES buffer;
[0135] 5) Add 0.05 mg of the insulin monoclonal antibody obtained in Example 1, then dilute to 1 mL with 50 mM MES buffer at pH 6.0, and mix well;
[0136] 6) React overnight at 37°C;
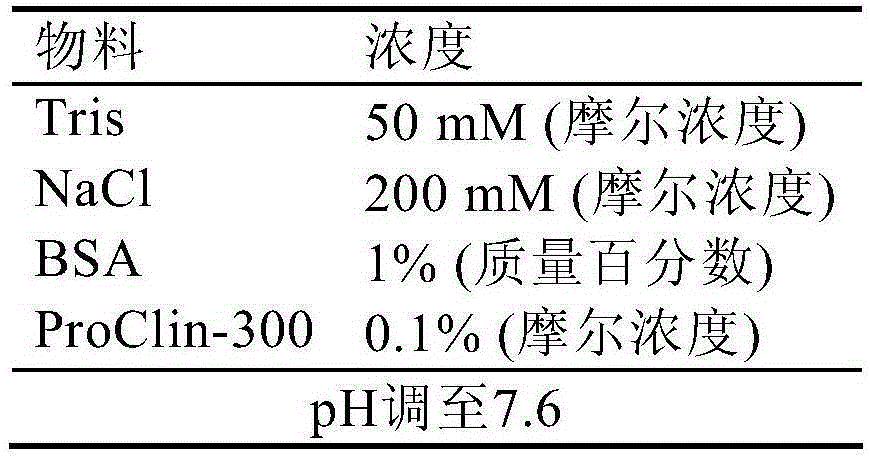
[0137] 7) Wash twice with a solution containing 50 mM pH7.8 Tris-HCl, 150 mM NaCl, and 1% BSA.
[0138]8) Add 2 mL of a solution containing 50 mM pH7.8 Tris-HCl, 150 mM NaCl, and 1% BSA, and mix well.
[0139] 9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| reaction rate constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com