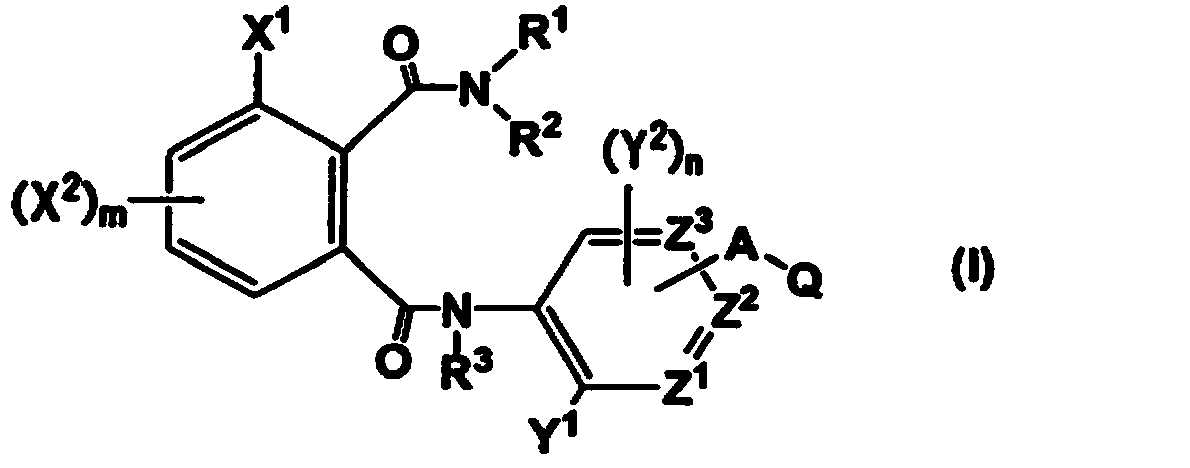
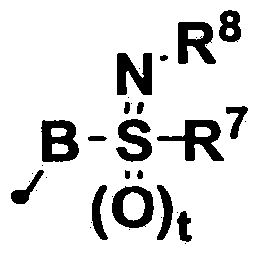Phthalamide derivative, pesticide for agricultural and horticultural applications which comprises said derivative, and method of utilizing said pesticide
A technology of phthalamide, derivatives, applied in the field of agricultural and horticultural pesticides, capable of solving problems such as damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
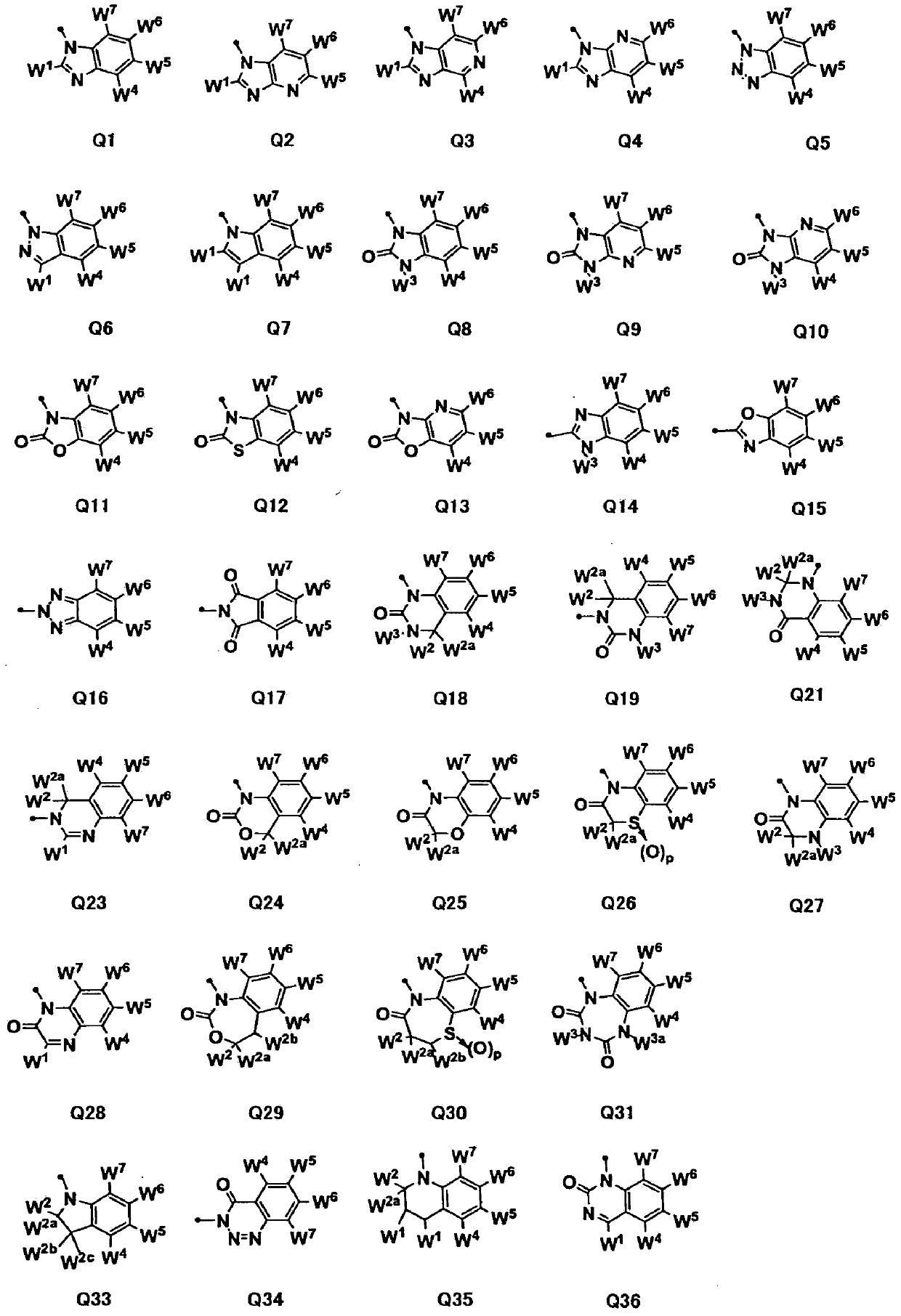
[0836] Preparation method 3-1. Preparation method of Q1'
[0837] [Chemical formula 11]
[0838]
[0839] (Where W 1 , W 4 , W 5 , W 6 And W 7 Same definition as above).
[0840] For the general formula Q1'(W 1 =CF 3 ), for example, 4-heptafluoroisopropyl-1,2-phenylenediamine (Qa) described in JP 2001-122836 A can be used in accordance with J. Am. Chem. Soc., 75 Volume, (1953), page 1292, to prepare 5-heptafluoroisopropyl-2-trifluoromethyl-1H-benzimidazole.
[0841] For the general formula Q1'(W 1 =NH 2 For the compounds of ), for example, 2-amino-5-heptafluoroisopropyl-1H-benzimidazole can be prepared from Qa according to the method described in J. Chem. Soc., 1960, page 2369.
[0842] For the general formula (Q1': W 1 =EtO) derivatives, for example, in accordance with the method described in J. Heterocycl. Chem., 28, (1991), page 933, 2-ethoxy-5-heptafluoroiso can be prepared from Qa in the same manner. Propyl-1H-benzimidazole.
[0843] Preparation method 3-2. Preparation method of Q...
Embodiment 1-1
[1313] [Chemical formula 26]
[1314]
[1315] 10 g of 1,2-phenylenediamine, 32.7 g of heptafluoro-2-iodopropane, 14.6 g of sodium carbonate and 1 g of tetrabutylammonium hydrogen sulfate were sequentially added to a mixed solution of 100 ml of ethyl acetate and 100 ml of water. Under stirring at room temperature, 16 g of sodium dithionite was added in small amounts over 30 minutes. After the addition, the temperature was raised to 40°C and stirred for 1 hour. After separating the organic layer, it was washed with saturated brine, and dried over magnesium sulfate. The solvent was distilled off under reduced pressure to obtain 24.3 g of 4-heptafluoroisopropyl-1,2-phenylenediamine.
[1316] 1 H-NMR(CDCl 3 ,ppm): 3.46(2H,brs), 3.61(2H,brs), 6.74(1H,d), 6.91(1H,d), 6.95(1H,s)
Embodiment 1-2
[1318] [Chemical formula 27]
[1319]
[1320] 2.76 g of 4-heptafluoroisopropyl-1,2-phenylenediamine was dissolved in 5 ml of trifluoroacetic acid, and the reaction was carried out under heating and reflux for 3 hours. After the reaction, the excess trifluoroacetic acid was distilled off under reduced pressure, and the obtained residue was washed with an ether-hexane mixed solvent to obtain 5-heptafluoroisopropyl-2-trifluoromethyl-1H-benzo Imidazole 3.1g. Physical properties: m.p.132-133°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com