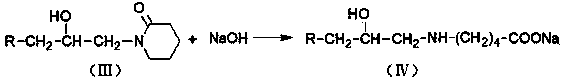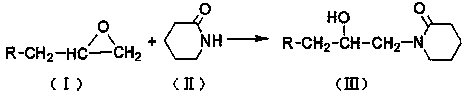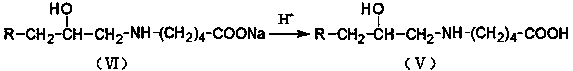Method for synthesizing beta-isoalkanol amino valeric acid cyclohexylamine
A technology of cyclohexylamine aminovaleric acid and a synthesis method, which is applied in the field of vapor phase antirust additive synthesis, can solve the problems of few product varieties and limited application coverage, achieve strong antirust effect, improve anodic polarization performance, and react Simple and easy to operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a four-neck flask equipped with a stirring device, a reflux condensing device and a thermometer, add 120 grams of toluene, add 1.12 grams of zinc chloride and 48 grams of valerolactam, slowly add 28 grams of alkylene oxide, and stir at 70 ° C Reflux reaction at ~75°C for 10 hours. After the reaction was completed, the solvent and unreacted substances were distilled off under reduced pressure to obtain a colorless liquid β-isoalkanol valerolactam. Add 20 178 grams of sodium hydroxide solution carry out hydrolysis reaction, obtain β-isoalkanol base sodium valerate solution, then slowly add dilute hydrochloric acid solution in β-isoalkanol base sodium valerate solution, adjust pH to about 1.0, The solid product was precipitated, washed back with water to obtain β-isoalkanol aminovaleric acid, and β-isoalkanol aminovaleric acid was added to 47.8 g of cyclohexylamine, stirred, heated to dissolve, and cooled to obtain β-isoalkanol aminovaleric acid Isoalkanolaminovaleric A...
Embodiment 2
[0023] In a four-neck flask equipped with a stirring device, a reflux condensing device and a thermometer, add 145 grams of toluene, add 1.32 grams of zinc chloride and 48.4 grams of valerolactam, slowly add 32 grams of alkylene oxide, and stir at 70 ° C Reflux reaction at ~80°C for 8 hours. After the reaction was completed, the solvent and unreacted substances were distilled off under reduced pressure to obtain a colorless liquid β-isoalkanol valerolactam. Add 20 178 grams of sodium hydroxide solution carry out hydrolysis reaction, obtain β-isoalkanol base sodium valerate solution, then slowly add dilute hydrochloric acid solution in β-isoalkanol base sodium valerate solution, adjust pH to about 1.0, The solid product was precipitated, washed back with water to obtain β-isoalkanol aminovaleric acid, and β-isoalkanol aminovaleric acid was added to 50.5 g of cyclohexylamine, stirred, heated to dissolve, and cooled to obtain β-isoalkanol aminovaleric acid. Isoalkanolaminovaleric...
Embodiment 3
[0025] In a four-neck flask equipped with a stirring device, a reflux condensing device and a thermometer, add 123 grams of toluene, add 2.4 grams of zinc chloride and 61.4 grams of valerolactam, slowly add 30 grams of alkylene oxide, and stir at 75 ° C Reflux reaction at ~80°C for 9 hours. After the reaction was completed, the solvent and unreacted substances were distilled off under reduced pressure to obtain a colorless liquid β-isoalkanol valerolactam. Add 20 178 grams of sodium hydroxide solution carry out hydrolysis reaction, obtain β-isoalkanol base sodium valerate solution, then slowly add dilute hydrochloric acid solution in β-isoalkanol base sodium valerate solution, adjust pH to about 1.0, The solid product was precipitated, washed back with water to obtain β-isoalkanol aminovaleric acid, and β-isoalkanol aminovaleric acid was added to 66.5 g of cyclohexylamine, stirred, heated to dissolve, and cooled to obtain β-isoalkanol aminovaleric acid. Isoalkanolaminovaleric ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com