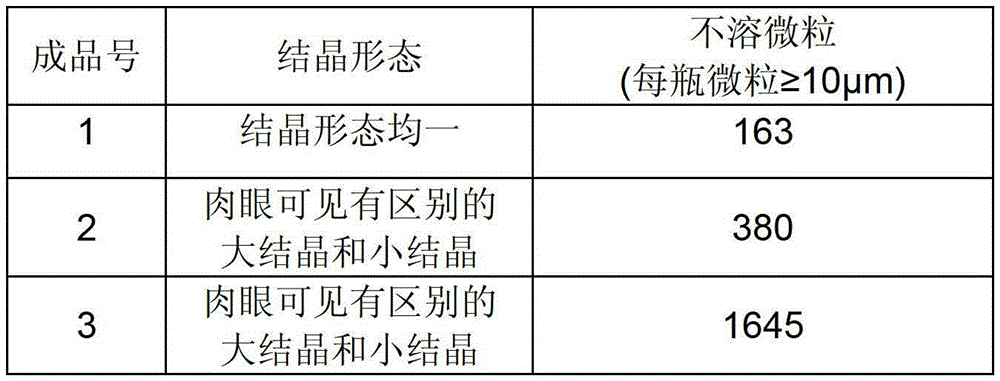
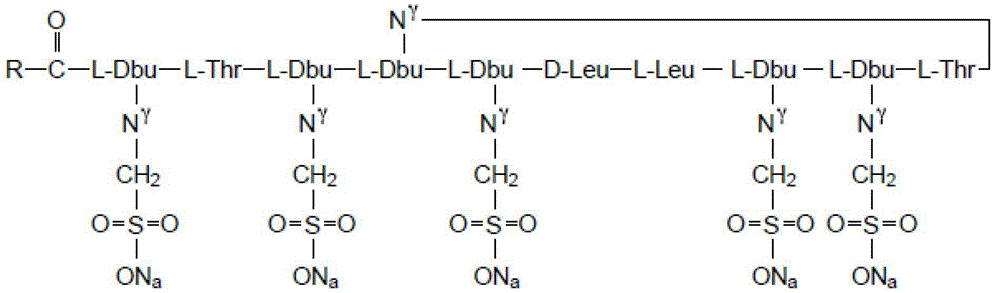A kind of polymyxin e sodium methanesulfonate freeze-dried preparation and preparation method thereof
A technology of sodium methanesulfonate and polymyxin, which is applied in the field of medicine, can solve the problems of large particle size, collapsed appearance of polymyxin E sodium methanesulfonate freeze-dried preparation, and many insoluble particles, and achieves uniform crystal form. , The appearance is full, the effect of easy rehydration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
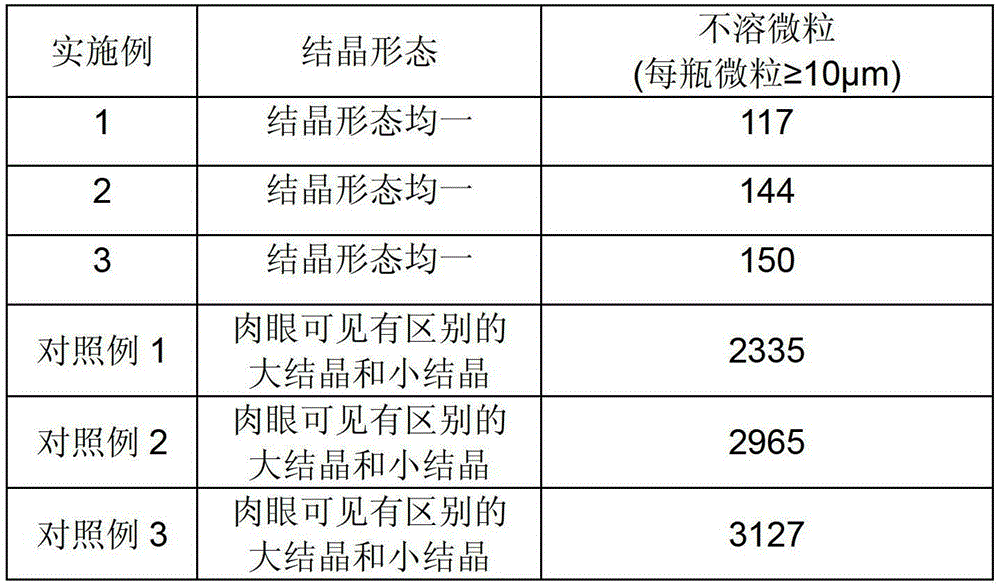
Embodiment 1
[0037] Sodium polymyxin E methanesulfonate 0.077g
[0038] Add water for injection to make 2ml solution
[0039] Add the prescribed amount of polymyxin E sodium methanesulfonate into the water for injection, stir and dissolve, pass through a 0.22 μm microporous filter membrane for sterile filtration, and fill the filtrate into 10ml vials with 2ml each aseptically, and half-tighten the stopper to the freeze dryer. Control the temperature of the front box of the freeze dryer at -30°C to -35°C, pre-freeze the product to this temperature and maintain it for 2 hours, and at the same time the temperature of the cold trap drops to -60°C; adjust the temperature of the partition to -15°C, when the product rises to this temperature Maintain the temperature for 2 hours; adjust the temperature of the partition to -30°C ~ -35°C again, and maintain it for 1 hour after the product drops to this temperature; turn on the vacuum pump, and then adjust the temperature of the partition to 0°C, wh...
Embodiment 2
[0041] Sodium polymyxin E methanesulfonate 0.155g
[0042] Add water for injection to make 2ml solution
[0043] Add the prescribed amount of polymyxin E sodium methanesulfonate into the water for injection, stir and dissolve, pass through a 0.22 μm microporous filter membrane for sterile filtration, and fill the filtrate into 10ml vials with 2ml each aseptically, and half-tighten the stopper to the freeze dryer. Control the temperature of the front box of the freeze dryer at -40°C to -45°C, pre-freeze the product to this temperature and maintain it for 3 hours, and at the same time the temperature of the cold trap drops to -60°C; adjust the temperature of the partition to -15°C, when the product rises to this temperature Maintain the temperature for 3 hours; adjust the temperature of the partition to -40°C ~ -45°C again, and maintain it for 1 hour after the product drops to this temperature; turn on the vacuum pump, and then adjust the temperature of the partition to 0°C, wh...
Embodiment 3
[0045] Sodium polymyxin E methanesulfonate 0.310g
[0046] Add water for injection to make 2ml solution
[0047] Add the prescribed amount of polymyxin E sodium methanesulfonate into the water for injection, stir and dissolve, pass through a 0.22 μm microporous filter membrane for sterilizing filtration, and the filtrate is aseptically filled in 10ml vials with 2ml each, and half-tightened to the freeze dryer. Control the temperature of the front box of the freeze dryer at -40°C to -45°C, pre-freeze the product to this temperature and maintain it for 3 hours, and at the same time the temperature of the cold trap drops to -60°C; adjust the temperature of the partition to -15°C, when the product rises to this temperature Maintain the temperature for 3 hours; adjust the temperature of the partition to -40°C ~ -45°C again, and maintain it for 2 hours after the product drops to this temperature; turn on the vacuum pump, and then adjust the temperature of the partition to 0°C, when...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com