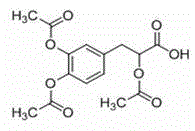
Acetyl tanshinol freeze-dried powder injection and preparation method thereof
A technology of freeze-dried powder injection and acetyldanshen, which is applied in the field of medicine, can solve the problems of poor stability and low solubility of acetyldanshensu, and achieve the effects of improved stability, excellent appearance, and small weight loss on drying
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the influence of cosolvent on the solubility of acetyldanshensu
[0023] In order to improve the solubility of acetyldanshensu and ensure its stability, it is necessary to add certain co-solvents in water, such as ethanol, PEG-400, 1,2-propylene glycol, sodium bicarbonate, disodium hydrogen phosphate, sodium citrate, spit One or more of Wen-80 and poloxamer.
[0024]
[0025] The results showed that the selected co-solvents could increase the solubility of acetyldanshensu to different degrees, and the content remained basically stable.
Embodiment 2
[0026] Embodiment 2: The influence of pH on the stability of acetyldanshensu
[0027] Weigh an appropriate amount of acetyldanshensu, add 20% ethanol and 15% PEG-400 to aid dissolution, and prepare a 50 mg / mL acetyldanshensu solution with water for injection, and use 0.1moL / L hydrochloric acid or 0.1moL / L sodium hydroxide solution respectively Adjust the pH to 3.5, 4.5, 5.0, 5.5, 6.0, 6.5, 7.5, and 8.5, and investigate the changes in the content of acetyldanshensu after being placed under different conditions for different periods of time. The results are shown in Table 2.
[0028]
[0029] The results showed that as the pH of the solution increased, the degradation of acetyldanshensu became faster and the color of the solution became darker. Combined with the pH range (4.0-9.0) required for intravenous injection, the pH range of the acetyldanshensu solution is selected to be 4.0-6.0, preferably 4.5-5.5.
Embodiment 3
[0030] Example 3: Effect of excipients on product formability
[0031] Choose 10% sodium citrate as co-solvent to prepare 15% acetyldanshensu solution. Mannitol and dextran were selected as excipients, and the following prescription was designed for lyophilization. The results are shown in Table 3.
[0032]
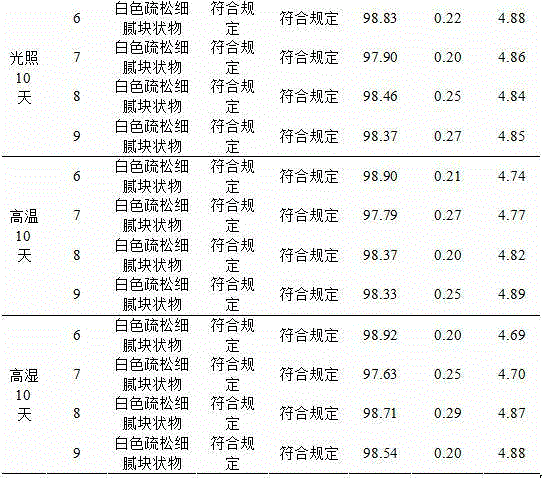
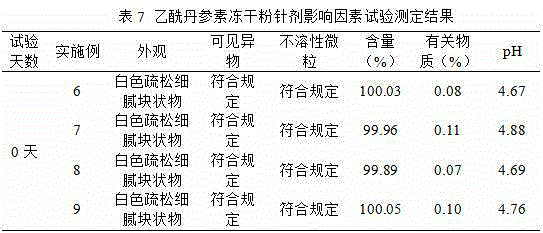
[0033] According to the results of the prescription screening test, when the prescription contains 10% sodium citrate, without adding excipients, it can be prepared into white fine and loose block freeze-dried products with complete skeleton and good formability, with uniform texture and good resolubility. Well, dissolve immediately (within 10 seconds) after adding water and shaking. Considering the cost saving of freeze-drying, when the prescription contains sodium citrate, there is no need to use excipients.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com