Protamine mimetic peptide and pharmaceutical salt and application thereof
A protamine and peptide-mimicking technology, which is applied in the field of biomedicine, can solve problems such as difficulty in achieving uniformity and consistency in quality, lack of substitute drugs for protamine, and restrictions on the production of protamine, so as to facilitate artificial synthesis and eliminate bleeding Tendency, the effect of reducing the sensitization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the design of protamine mimetic peptide
[0037] Design ideas and methods: Heparin is a natural anticoagulant substance extracted from bovine lung or pig small intestinal mucosa. Its average molecular weight is 15KD mucopolysaccharide sulfate, which is strongly acidic and has a large amount of negative charge. According to the characteristics of the chemical properties of heparin, the present invention first uses a polypeptide composed of positively charged basic amino acids to neutralize or antagonize heparin with a large amount of negative charge.
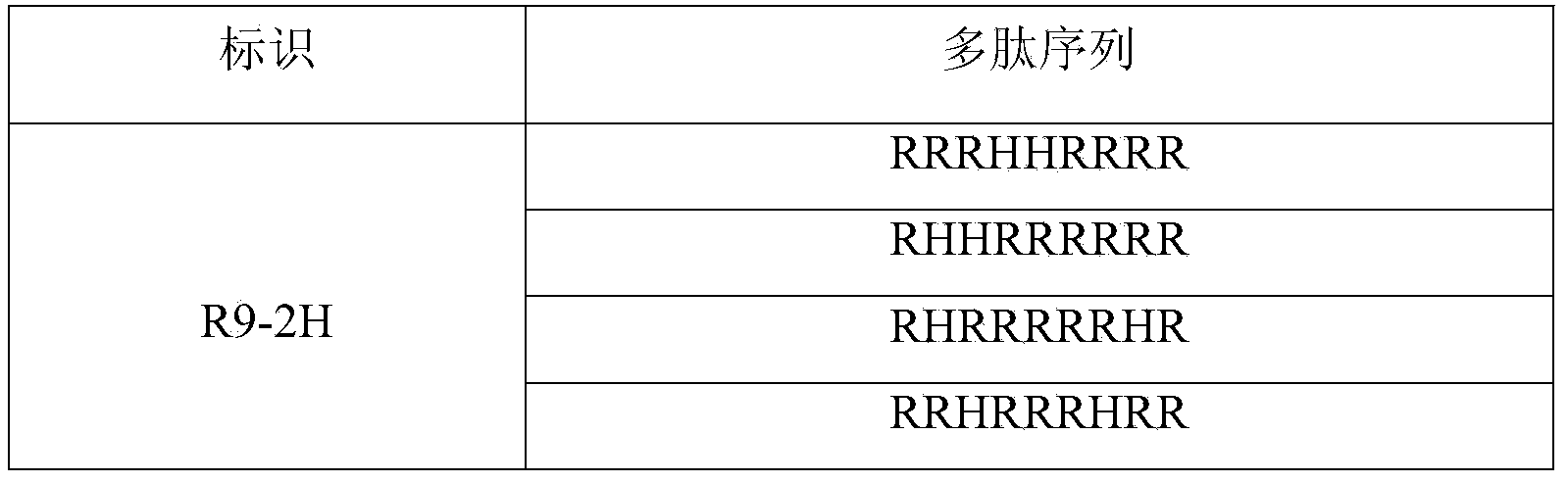
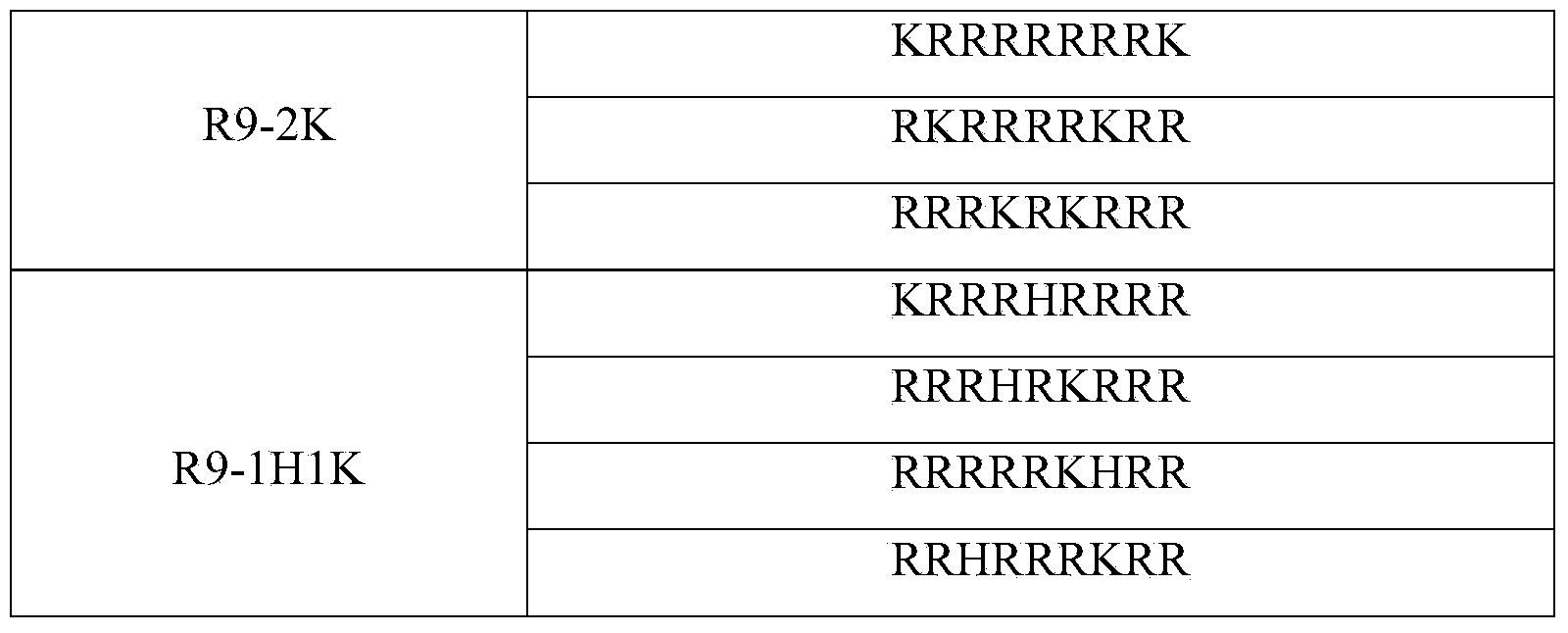
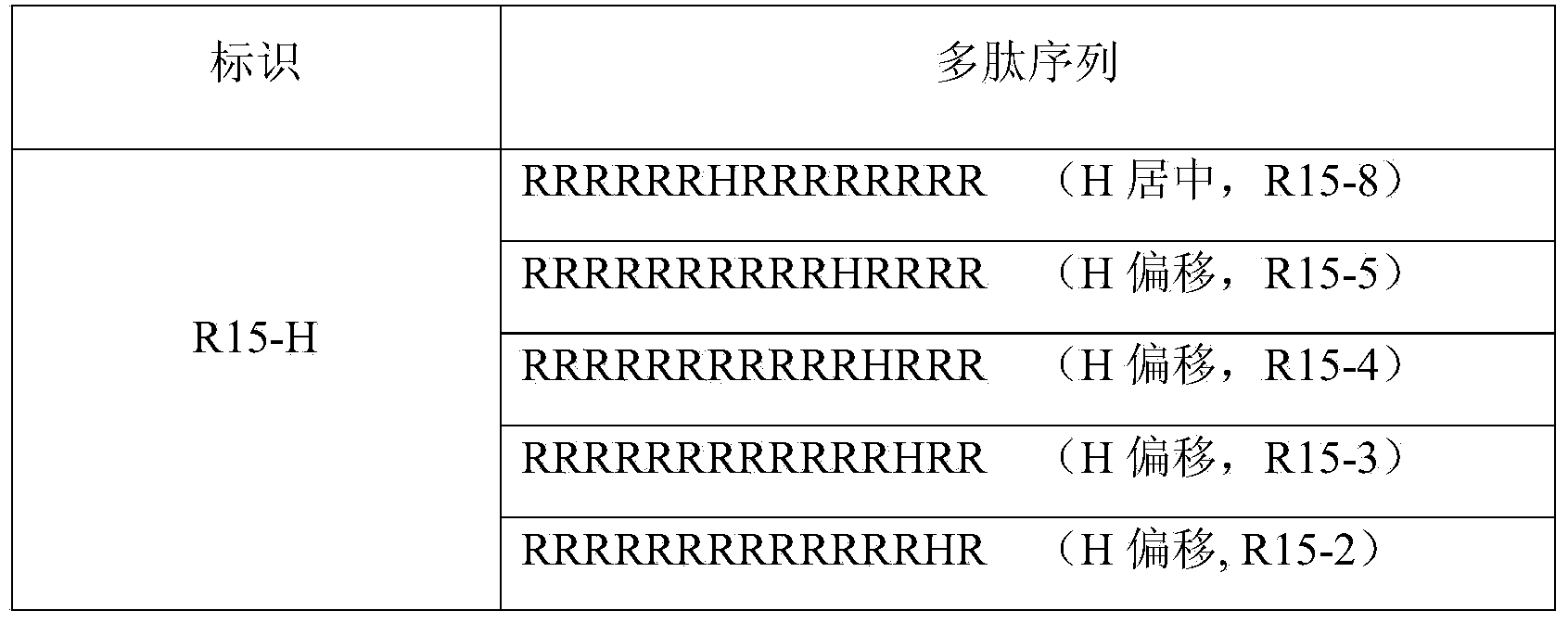
[0038] Arranged according to the strength of basicity, the three basic amino acids are arginine (Arg, R) > lysine (Lys, K) > histidine (His, H). Through theoretical design and effective experimental verification, the present invention finally determines a series of artificially designed and artificially synthesized protamine mimetic peptides. They are linear polypeptides with 9 to 20 amino acids consisting o...
Embodiment 2
[0064] Embodiment 2: the synthesis of protamine mimetic peptide
[0065] In 1963, American scientist R.B. Merrifield invented the method of immobilizing the carboxyl terminal (C terminal) of the amino acid of the target peptide on an insoluble resin, and the amino terminal (N terminal) of the amino acid bound to the resin is condensed with the carboxyl terminal of the amino acid to be connected. Solid-phase synthesis to elongate peptide chains. The sequence of peptide synthesis starts from the carboxy-terminal (C-terminal) of the polypeptide and extends one by one in the direction of the amino-terminal (N-terminal) of the peptide. When carrying out the carboxyl conjugation reaction of amino acid, its amino group and side chain group should be protected to avoid the reaction. At present, tert-butoxycarbonyl (Boc) protection method and fluorenylmethyloxycarbonyl (Fmoc) protection method are commonly used. Therefore, every To connect an amino acid, it needs to undergo a condensa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com