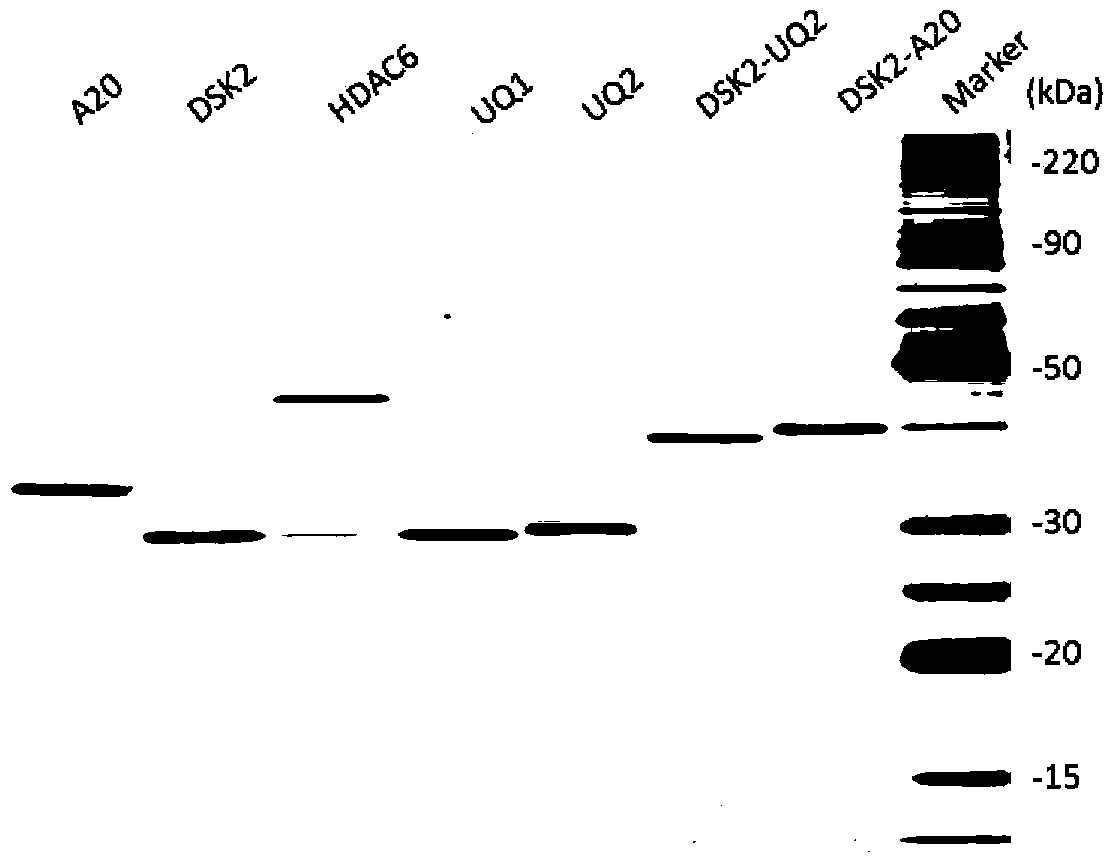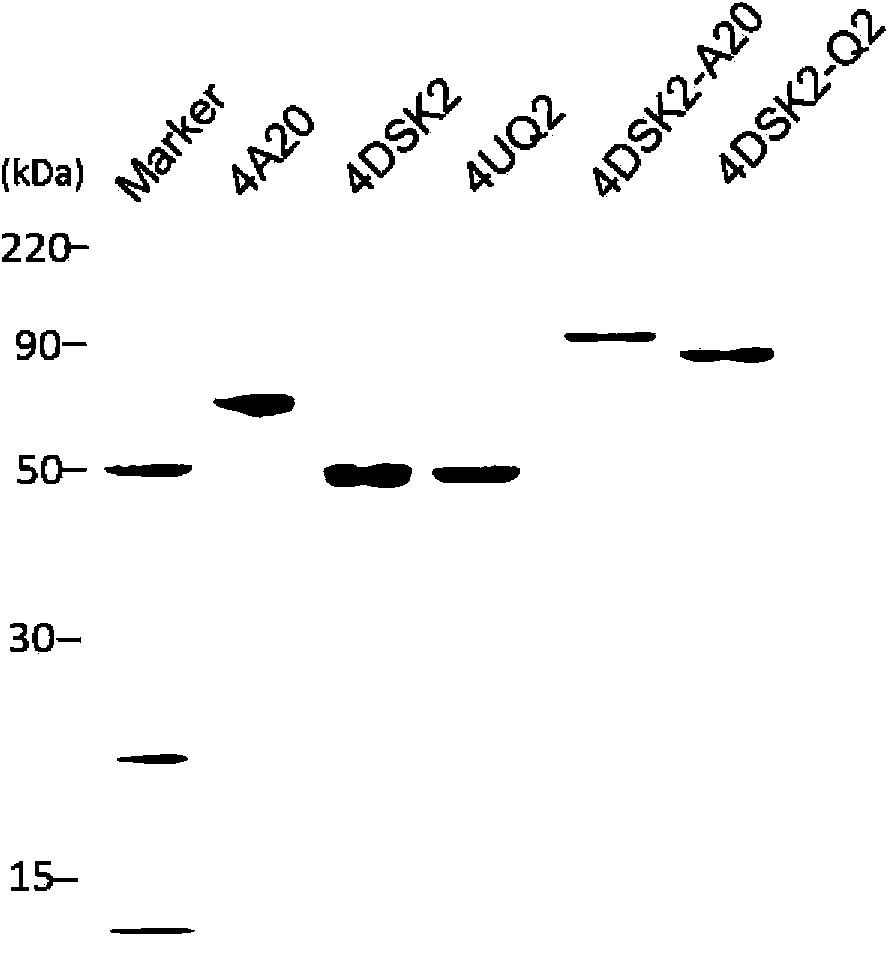Artificially constructed hybrid ubiquitin conjugation structure domain polypeptide and application thereof
A technology that combines structural domains and structural domains, applied in the biological field, can solve problems such as the inability to comprehensively ubiquitinate proteomics research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1. Quantitative comparison of affinity properties between different ubiquitin-binding domains and ubiquitin.
[0084] 1. Expression and purification of ubiquitin-binding domain polypeptides
[0085] 1. Construction of recombinant expression vector
[0086] DNA fragments containing different ubiquitin-binding domains were amplified by PCR method, and cloned into pGEX-4T-2 expression vector (GE Life Sciences) containing GST-tagged protein. The cloned ubiquitin binding domain is fused with the GST tag protein to obtain the GST-ubiquitin binding domain fusion protein. In addition, a hybrid ubiquitin-binding domain was also constructed: two different ubiquitin-binding domain coding sequences were connected through a linker sequence using fusion PCR technology to form a hybrid ubiquitin-binding domain, and it was cloned into the GST-tagged protein on the pGEX-4T-2 expression vector. See Table 1 for information about the ubiquitin-binding domains involved in this ex...
Embodiment 2
[0125] Example 2. Quantitative comparison of the affinity properties of different ubiquitin-binding domains generated in tandem with ubiquitin
[0126] 1. Expression and purification of tandem ubiquitin-binding domain polypeptides
[0127] 1. Construction of recombinant expression vector
[0128] There are five types of tandem ubiquitin binding domains involved in this example: 4UQ2, 4DSK2, 4A20, 4(DSK2-A20) and 4(DSK2-UQ2). Each hybrid ubiquitin-binding domain was cloned into the pGEX-4T-2 vector by a method similar to that of DSK2 in Step 1 of Example 1 to obtain corresponding recombinant plasmids. The specific coding sequence of each ubiquitin-binding domain is as follows: 4UQ2 ubiquitin-binding domain (position 859-1008 of sequence 4+GGTGGAGGTGGATCTGGTGGAGGT+position 859-1008 of sequence 4+GGTGGAGGTGGATCTGGTGGAGGT+position 859-1008 of sequence 4+GGTGGAGGTGGATCTGGTGGAGGT+sequence 859-1008 of sequence 4), 4DSK2 ubiquitin binding domain (709-843 of sequence 3+GGTGGAGGTGGATC...
Embodiment 3
[0163] Example 3. Comparing the affinity characteristics of hybrid ubiquitin binding domains with different numbers in series and ubiquitin
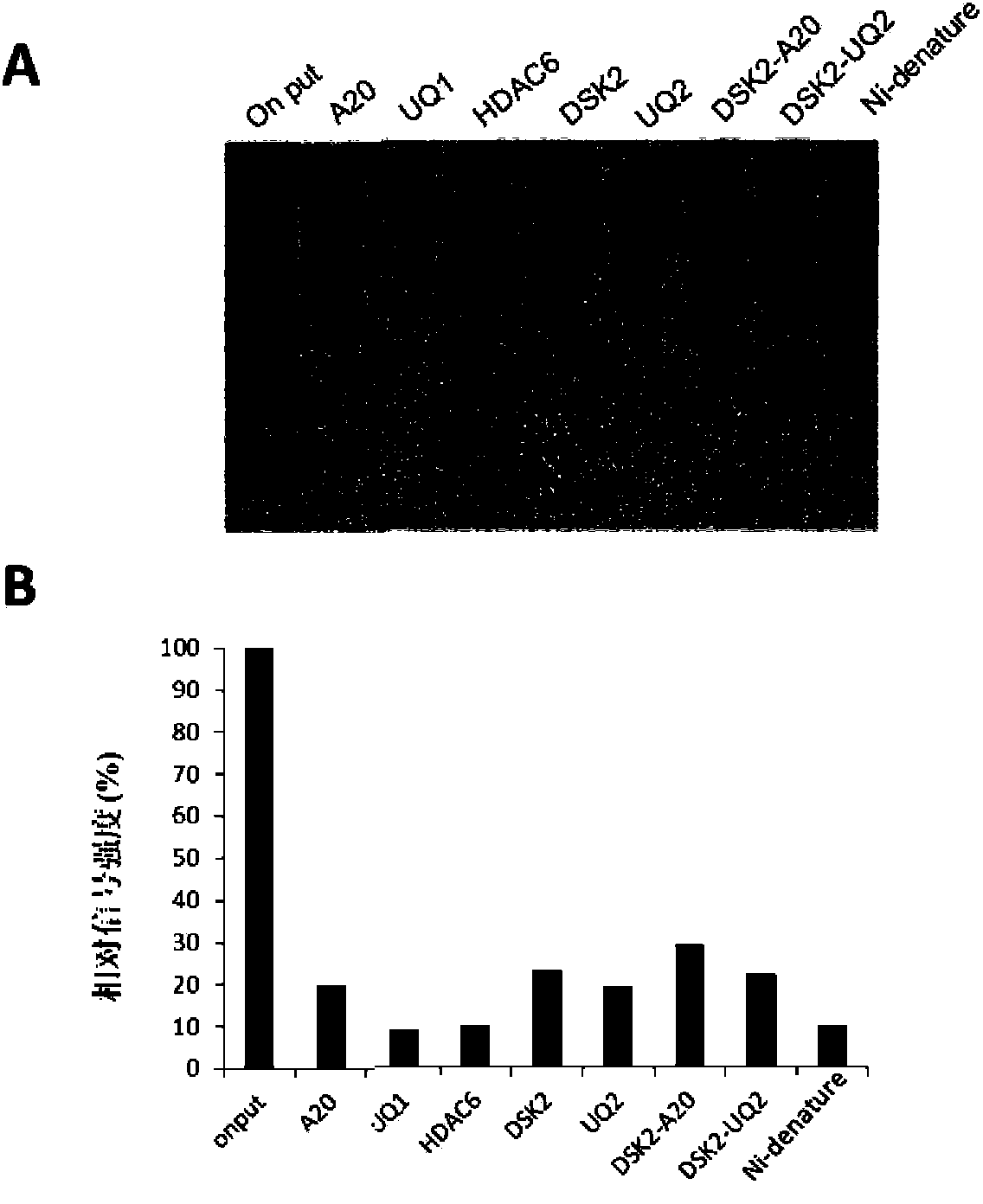
[0164] In order to further screen for more efficient ubiquitinated protein enrichment and purification materials, the inventors of the present invention compared the affinity between hybrid ubiquitin binding domains and ubiquitin chains with different serial numbers.
[0165] 1. Expression and purification of hybrid ubiquitin-binding domain polypeptides with different numbers in tandem
[0166] 1. Construction of recombinant expression vector
[0167] There are three types of tandem ubiquitin binding domains involved in this example: DSK2-A20, 2(DSK2-A20) and 4(DSK2-A20). Each hybrid ubiquitin-binding domain was cloned into the pGEX-4T-2 vector by a method similar to that of DSK2 in Step 1 of Example 1 to obtain corresponding recombinant plasmids. The specific coding gene sequence of each ubiquitin binding domain is as follows: DSK2-A2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com