Composite material, preparation method of composite material, and lithium ion battery comprising composite material
A composite material, lithium source technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of organic consumption, increased cost, and insufficient connection, achieving obvious cost-effective advantages, improved pressure resistance, and good consistency Effects of Sex and Uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] An embodiment of the present invention provides a composite material, the composite material is a multi-layer core-shell structure, the shell layer of the composite material includes a middle layer and an outer layer coated outside the middle layer, and the inner core of the composite material is Li(Ni x co y Al z )O 2 , the middle layer is Li(Ni 1 / 3 co 1 / 3 mn 1 / 3 )O 2 , the outer layer is LiNi 0.5 mn 1.5 o 4 , the mol ratio of the inner core, the middle layer and the outer layer of the composite material is a:b:c, wherein a+b+c=1, 0
[0045] The composite material provided by the embodiment of the present invention has a multi-layer core-shell structure. NCA material is selected for the inner core, NCM material is selected for the middle shell layer, and spinel lithium nickelate material is selected for the outer shell layer. Since the shell material itself has a certain capacity, the coating on the high-capacit...
Embodiment 2
[0048] The embodiment of the present invention provides a preparation method of a composite material, the preparation method comprising:
[0049] Step 1: Obtain a composite material precursor with a core-shell structure through co-precipitation in sequence. The shell layer of the composite material precursor includes an intermediate layer and an outer layer coated outside the intermediate layer. The core of the composite material precursor is (Ni x co y Al z )(OH) 2 , the middle layer is (Ni 1 / 3 co 1 / 3 mn 1 / 3 )(OH) 2 , the outer layer is Ni 0.25 mn 0.75 (OH) 2 ;
[0050] Step 2: Mix the precursor of the composite material with the lithium source according to the molar ratio of 1:1-1.1 and then roast, then cool, crush, and sieve to obtain the inner core as Li(Ni x co y Al z )O 2 , the middle layer is Li(Ni 1 / 3 co 1 / 3 mn 1 / 3 )O 2 , the outer layer is LiNi 0.5 mn 1.5 o 4 The composite material, the molar ratio of the inner core, the middle layer and the outer...
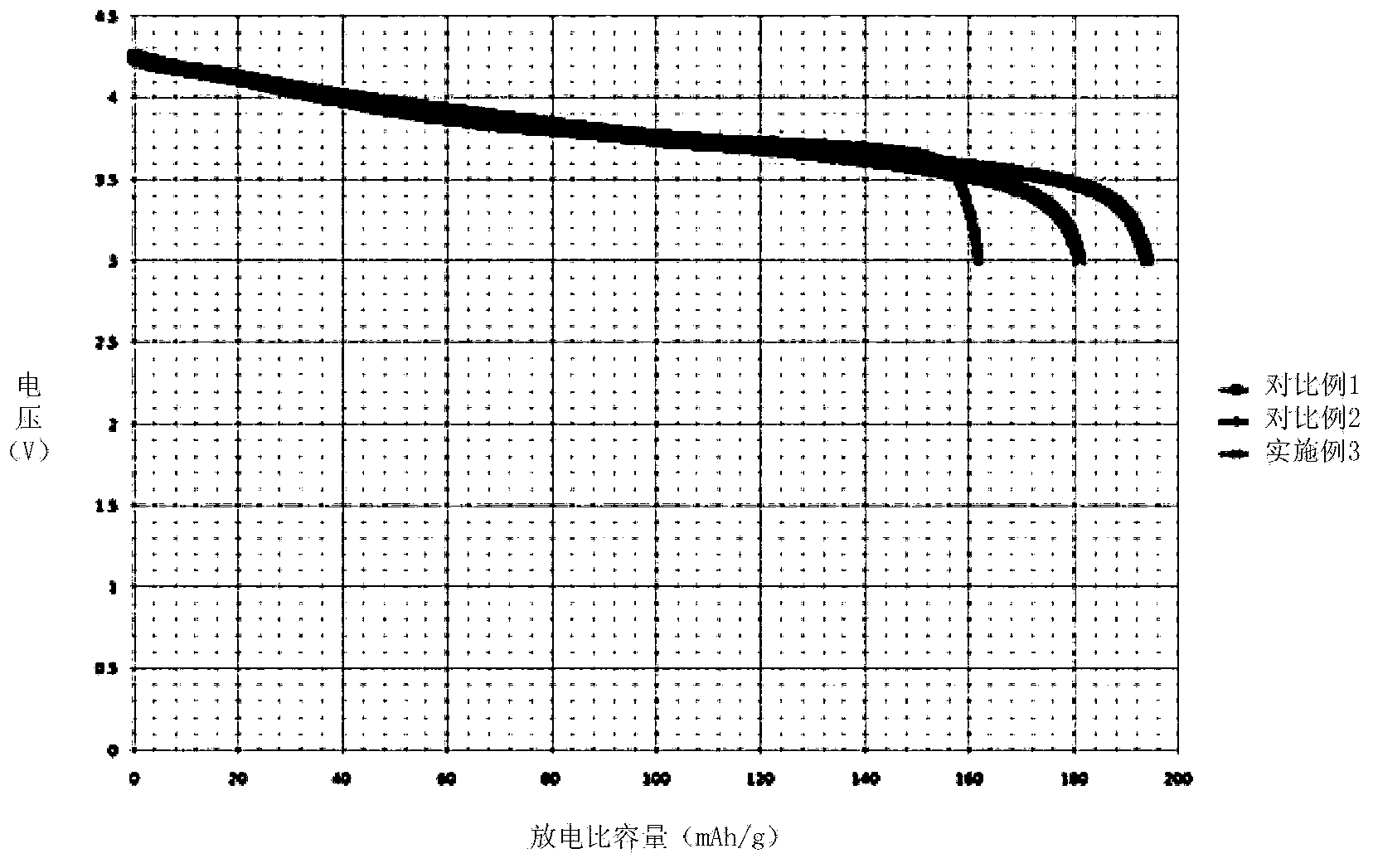
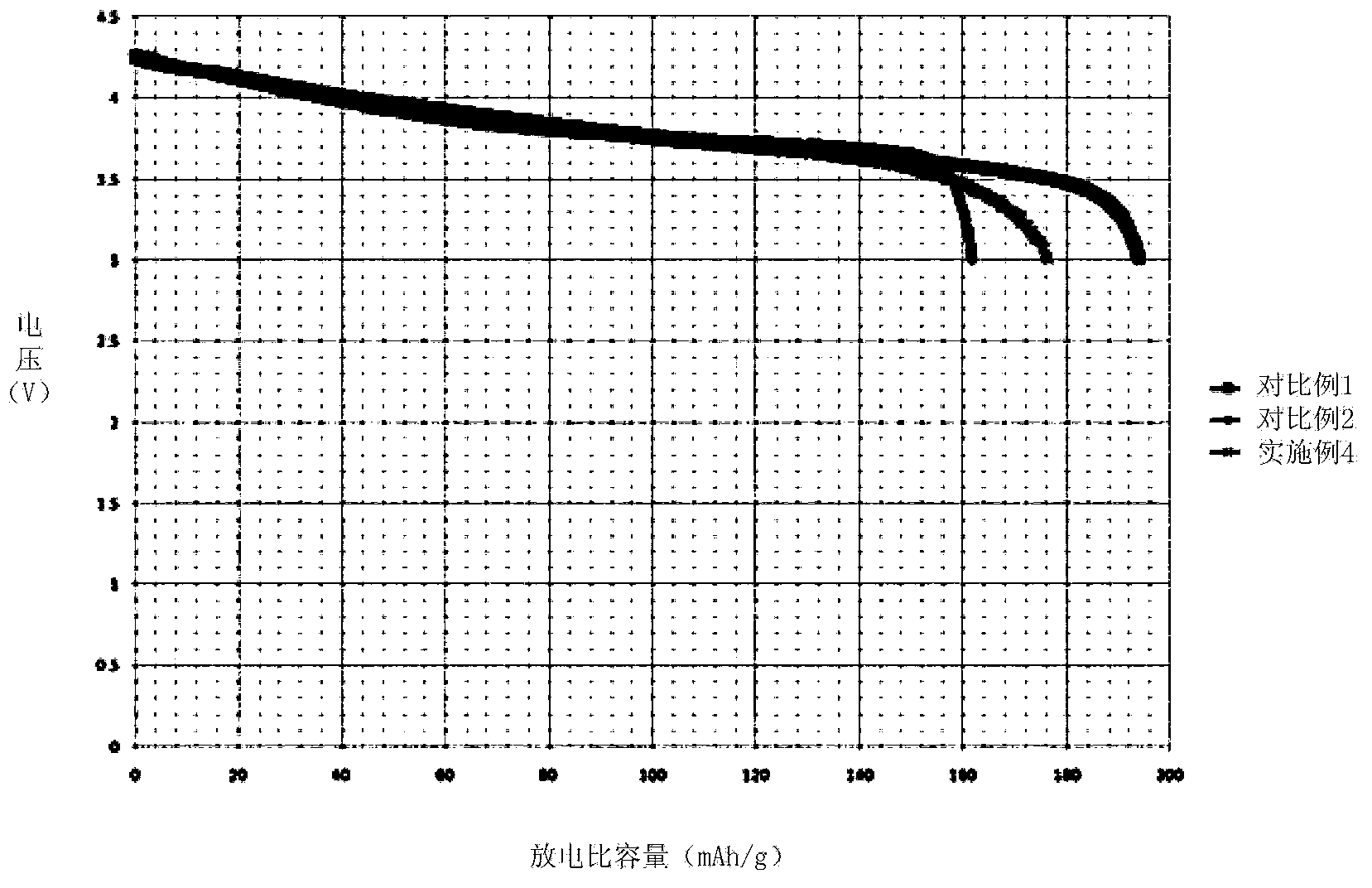
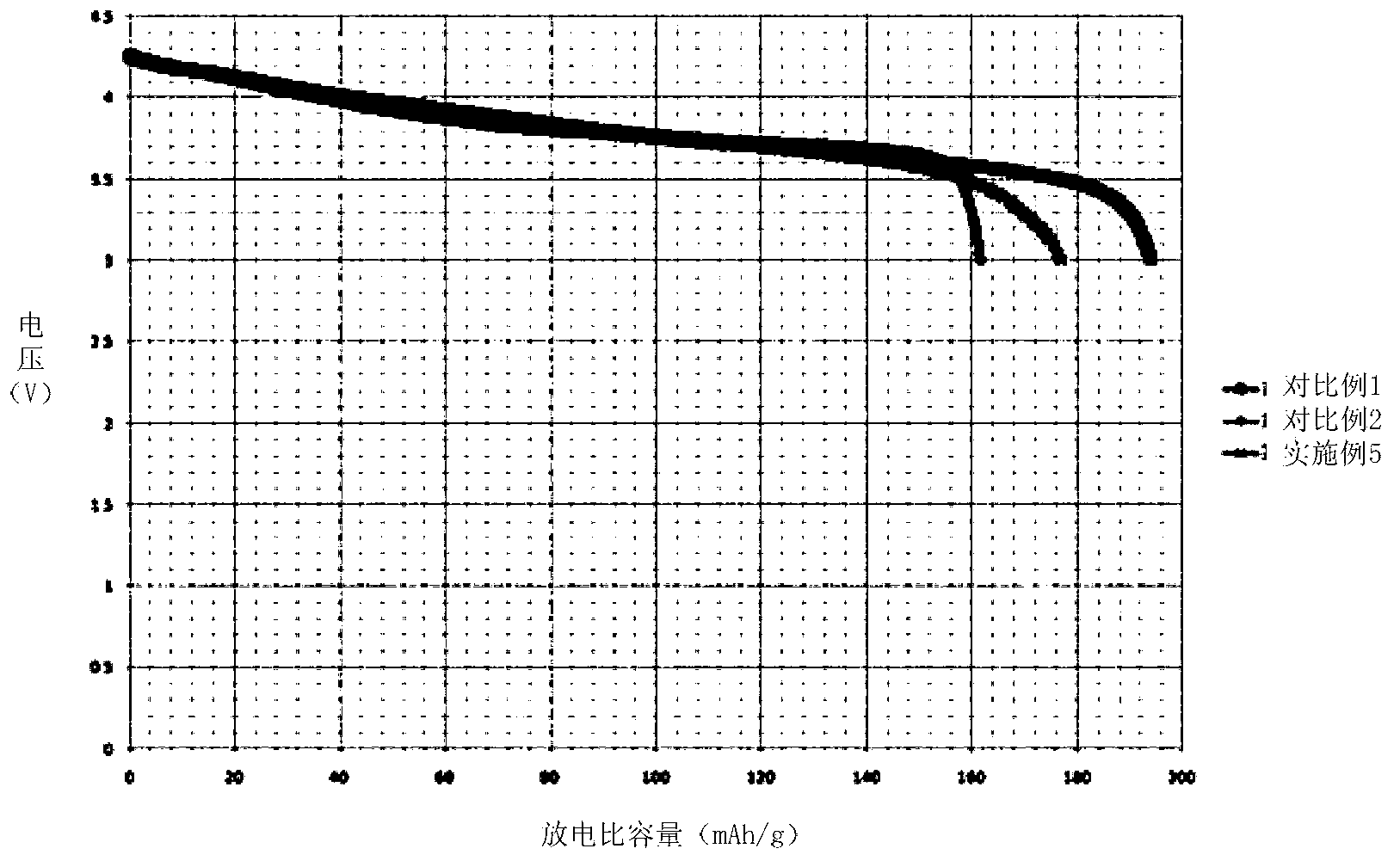
Embodiment 3
[0069]Prepare 9L of Ni, Co, and Al nitrate solution A with a concentration of 1mol / L, wherein the molar ratio of Ni, Co, and Al is 0.8:0.15:0.05; prepare 0.05L of Ni, Co, and Mn nitric acid with a concentration of 1mol / L Salt solution B, wherein the molar ratio of Ni, Co, and Mn is 1:1:1; preparing 1 L of Ni, Mn nitrate solution C with a concentration of 1mol / L, wherein the molar ratio of Ni, Mn is 0.25:0.75; Prepare a sufficient amount of 6mol / L NaOH alkali solution.
[0070] Inject solution A at a speed of 1L / h into a reaction kettle with a rotation speed of 200rps, and at the same time inject 6mol / L NaOH alkali solution to keep the pH value in the reaction kettle between 10-11. After the solution A is added dropwise, Immediately add solution B dropwise to the reactor at a rate of 0.5L / h. After 0.1h, solution B is completely injected into the reactor, and then immediately add solution C dropwise to the reactor at a rate of 0.5L / h. Solution C drops After the addition is comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com