Application of histone deacetylase inhibitor in preparation of latent virus activator
A technology of deacetylase and histone, applied in the field of microbiology, can solve the problems of latent and continuous infection in patients with chronic hepatitis C
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1. Effect of chidamide on the growth of HIV latently infected cells
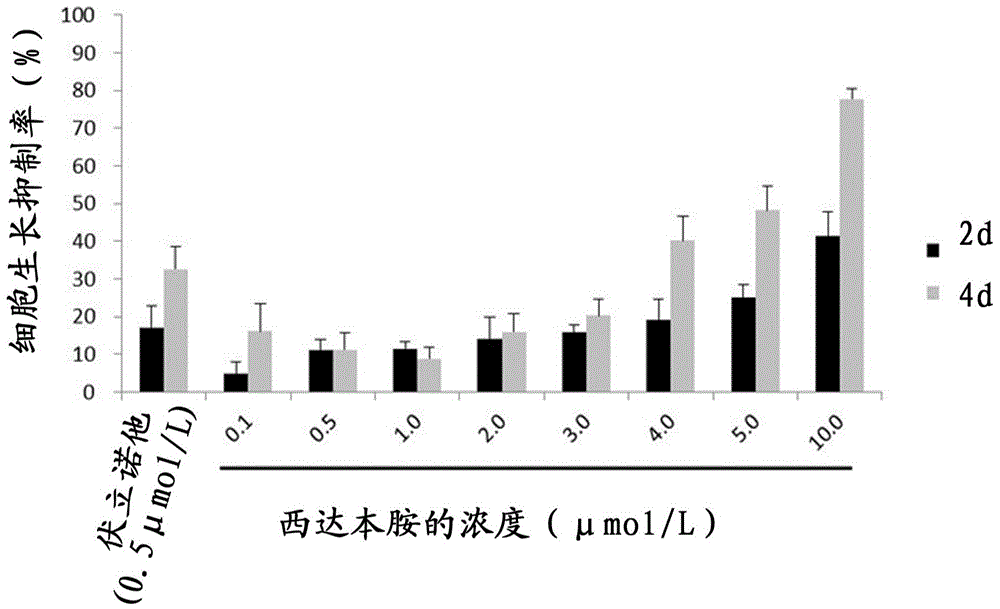
[0040] In this example, the MTT cell proliferation assay was used to detect the effect of the HDACi drug chidamide on the growth of virus latently infected cells A7, to confirm the cytotoxicity of chidamide and the concentration range for reactivating the latently infected virus.
[0041] 1.1 Experimental method
[0042] 1.1.1 Cell lines:
[0043] The HIV pseudovirus latent infection cell line J-Lat Tat-GFP Clone A7 (hereinafter referred to as A7) was donated by Dr. Eric Verdin, NIH AIDS Research and Standard Reagent Project Team, NIH AIDS Department, USA. A7 cells are human Jurkat T cells stably integrated with LTR-Tat-IRES-GFP via a viral vector. LTR (long terminal repeats) functions as a promoter and enhancer during viral transcription; the protein encoded by Tat gene can be combined with LTR to increase the transcription rate of viral genes; GFP (Green Fluorescent Protein) is a sign of H...
Embodiment 2
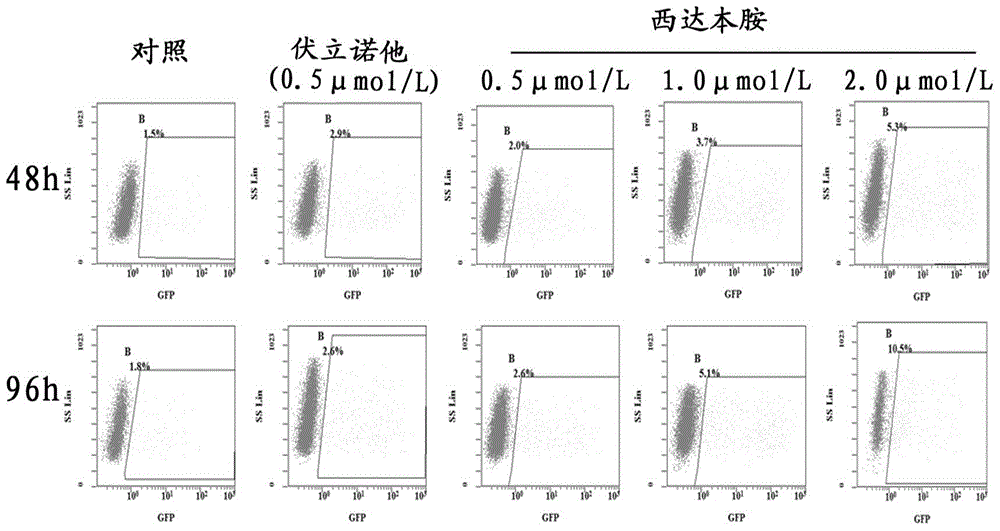
[0049] Example 2. Chidamide activates the HIV promoter of pseudovirus latent infection cell model A7
[0050] In this example, the HIV pseudovirus latently infected cell model A7 was used to prove the reactivation effect of chidamide and other HDACi on HIV virus promoters, and to explore the possibility of HDACi being used to prepare reactivation drugs for the in vivo storage of persistently infected viruses. See Example 1 for a description of A7 cells.
[0051] 2.1 Experimental method
[0052] The HIV pseudovirus latently infected cells A7 in the logarithmic growth phase were inoculated in a 12-well culture plate, and 1 × 10 6 cell. Then, different concentrations of chidamide were added to each well, the concentrations were: 0.5 μmol / L, 1.0 μmol / L, 2.0 μmol / L, 0.5 μmol / L vorinostat was used as a positive control, and no drug treatment was used. group is a negative control. 5% CO at 37°C 2 Cells were collected after cultured for 48h and 96h respectively, and the percentag...
Embodiment 3
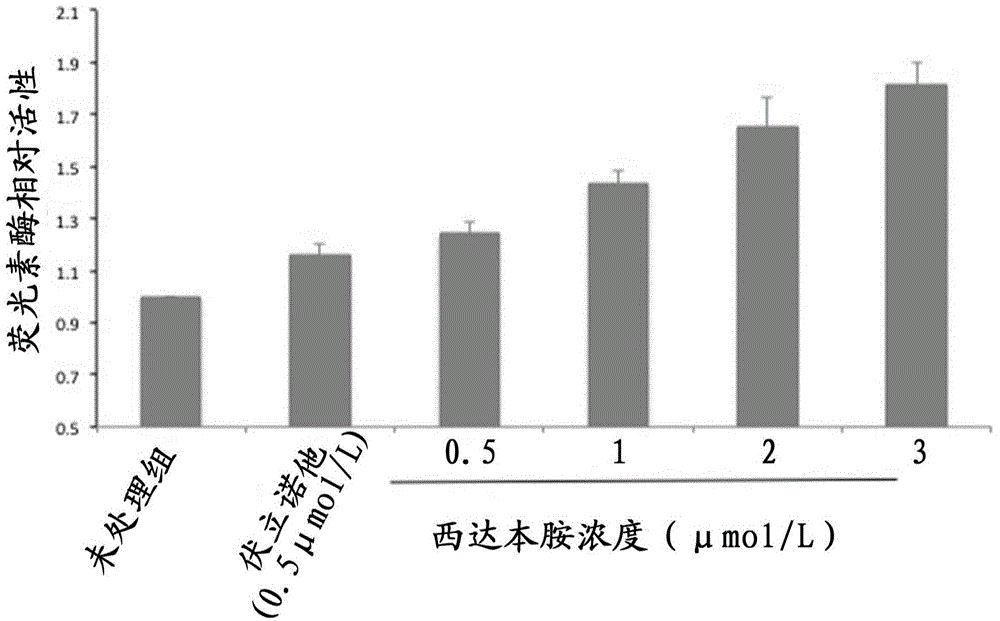
[0056] Embodiment 3.HDACi activates the HIV promoter of pseudovirus latent infection cell model TZM-bl
[0057] In this example, TZM-bl, another HIV pseudovirus latently infected cell model, is used to further demonstrate the reactivation effect of Chidamide and other HDACi on HIV virus promoters.
[0058] 3.1 Experimental method
[0059] 3.1.1 Cell lines:
[0060] The HIV pseudovirus latent infection cell model TZM-bl cell was donated by the NIH AIDS Research and Standard Reagent Project Group of the NIH AIDS Department in the United States. It is a human Hela cell that stably integrates the HIV LTR promoter and the luciferase gene (Luc). When the HIV promoter When activated, luciferase is produced in the cells and can be analyzed with a luciferase assay kit. The culture medium of TZM-bl cells is DMEM medium supplemented with 10% FBS.
[0061] 3.1.2 Experimental scheme:
[0062] The HIV pseudovirus latently infected cells TZM-bl in the logarithmic growth phase were inoculat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com