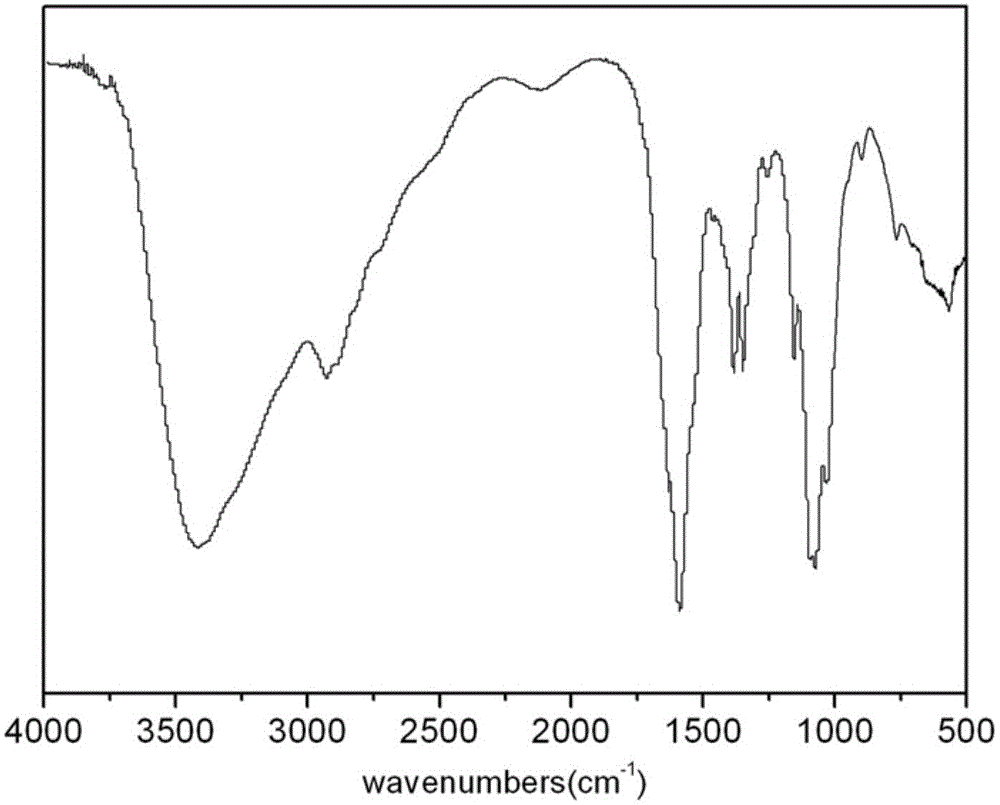
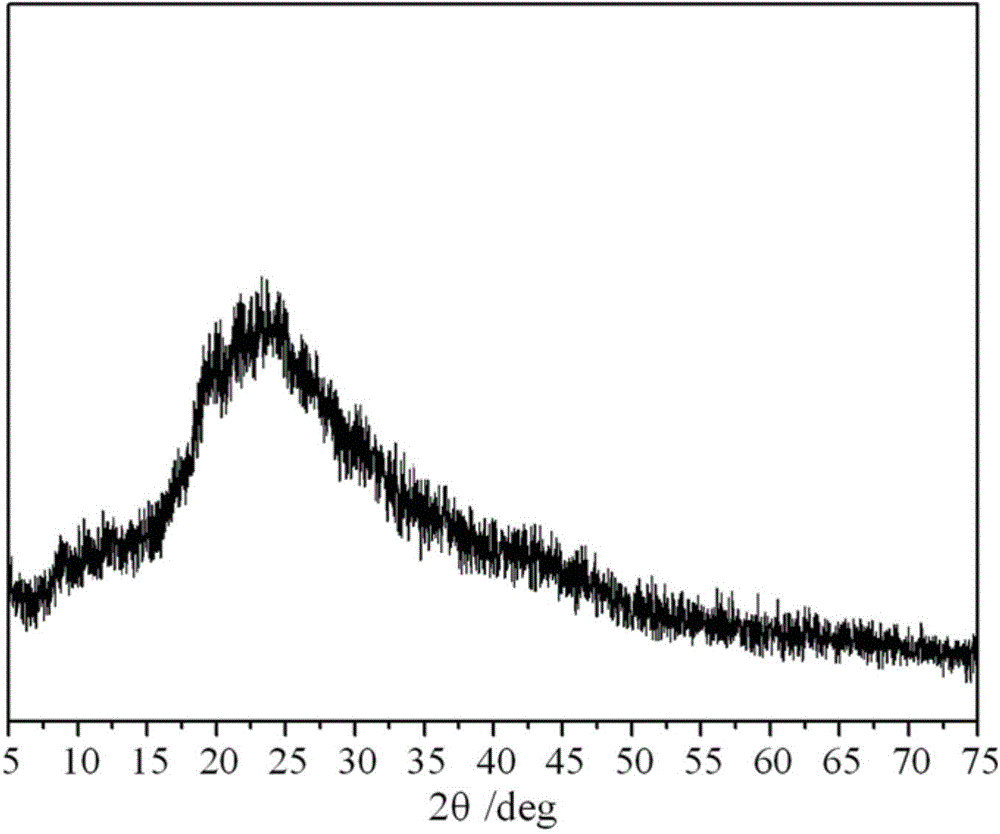
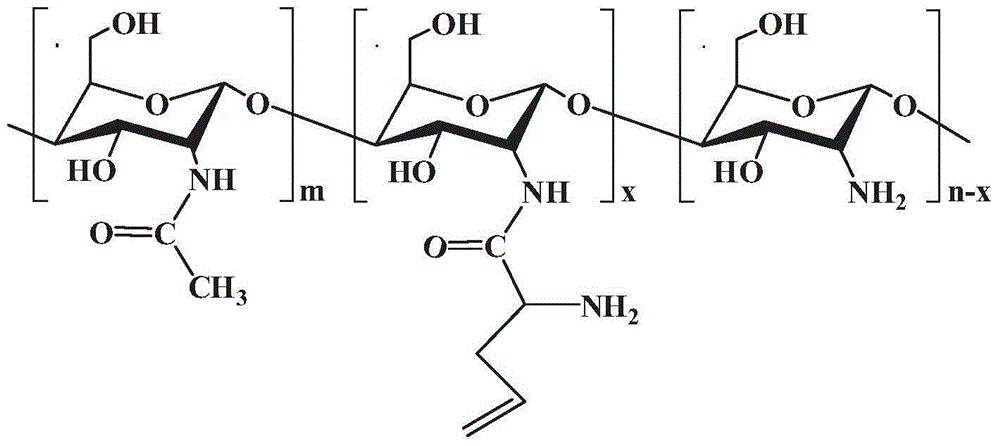
Polymerizable chitosan derivative and preparation method thereof
A chitosan derivative, chitosan technology, applied in the field of chitosan modification, can solve problems such as limiting the application range of chitosan, and achieve the effects of broadening the modification range, easy control, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Mix benzaldehyde and methanol at a volume ratio of 4% to obtain solution A; dissolve 2-amino-4-pentenoic acid in water to obtain a 4wt% aqueous solution, then add 80 mL of methanol and stir to obtain solution B; The molar ratio of benzaldehyde in solution B to 2-amino-4-pentenoic acid in solution B is 2: 1. Add solution A dropwise to solution B, stir while adding, and react at a constant temperature of 60°C after the addition is completed. 24h, rotary evaporated to precipitate, washed repeatedly with deionized water and methanol for 3 times, and dried at 60°C to obtain intermediate product a; chitosan with a weight average molecular weight of 3000 and a deacetylation degree of 90% was added to 1 wt% aqueous acetic acid , stirred at room temperature for 2h until chitosan was completely dissolved to obtain solution C; intermediate product a, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) was Added to solution C at a ratio of 1:3 and stirred at room temp...
Embodiment 2
[0025] Mix benzaldehyde and methanol at a volume ratio of 3% to obtain solution A; dissolve 2-amino-4-pentenoic acid in water to obtain a 5wt% aqueous solution, then add 60 mL of methanol and stir to obtain solution B; The molar ratio of benzaldehyde and 2-amino-4-pentenoic acid in solution B is 3:2, add solution A dropwise to solution B, stir while adding, and react at a constant temperature of 60°C after dropping 24h, rotary evaporated to precipitate, washed repeatedly with deionized water and methanol for 3 times, and dried at 60°C to obtain intermediate product a; chitosan with a weight average molecular weight of 12000 and a deacetylation degree of 80% was added with 1 wt% aqueous acetic acid , stirred at room temperature for 3h until chitosan was completely dissolved to obtain solution C; intermediate product a, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) was Ratio 2: 3 was added to solution C and stirred at room temperature for 4 h, wherein the amo...
Embodiment 3
[0027] Mix benzaldehyde and methanol at a volume ratio of 5% to obtain solution A; dissolve 2-amino-4-pentenoic acid in water to obtain a 5wt% aqueous solution, then add 70mL of methanol and stir to obtain solution B; The molar ratio of benzaldehyde and 2-amino-4-pentenoic acid in solution B is 3:1, add solution A dropwise to solution B, stir while adding, and react at a constant temperature of 60°C after the addition is completed 24h, rotary evaporated to precipitate, washed repeatedly with deionized water and methanol for 3 times, and dried at 60°C to obtain intermediate product a; chitosan with a weight average molecular weight of 50,000 and a deacetylation degree of 85% was added to 2wt% acetic acid aqueous solution , stirred at room temperature for 3h until chitosan was completely dissolved to obtain solution C; intermediate product a, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) was Added to solution C at a ratio of 2:1 and stirred at room temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com