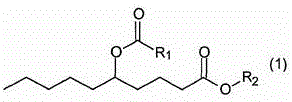
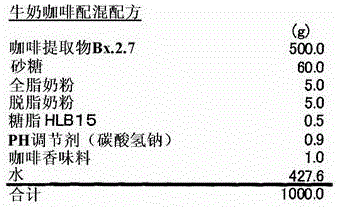
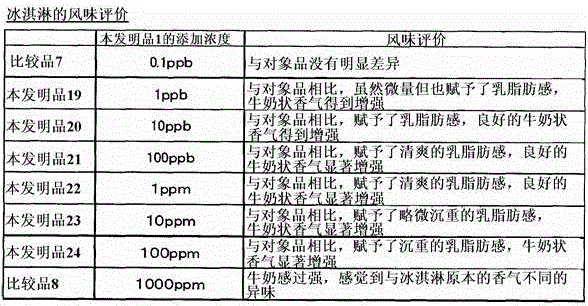
Capric acid derivatives and fragrance compositions
A composition and fragrance technology, applied in essential oils/fragrances, food science, fat production, etc., can solve the problem of satisfactory aroma and achieve a variety of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: the synthesis of methyl 5-hydroxydecanoate
[0051] Under a nitrogen atmosphere, δ-decalactone (manufactured by Sigma-Aldrich Japan K.K., 17.0 g, 0.10 mol) and methanol (190.0 g) were put into a 500 mL four-neck flask, and sodium methoxide (Junsho Chemical Co., Ltd. Co., Ltd., 28% methanol solution, 3.3 g, 0.017 mol). After stirring overnight at room temperature, glacial acetic acid (1.0 g, 0.017 mol) was added for neutralization, and concentrated under reduced pressure. Saturated brine (100 mL) was added to the obtained residue, followed by extraction with ether (200 mL), and the obtained ether layer was washed sequentially with saturated aqueous sodium bicarbonate (100 mL) and saturated brine (100 mL). By drying over anhydrous magnesium sulfate and concentrating under reduced pressure, 5-hydroxydecanoic acid methyl ester (18.0 g) was obtained as a colorless oily crude product. The yield was 89%.
Embodiment 2
[0052] Embodiment 2: the synthesis of methyl 5-formyloxydecanoate
[0053] Methyl 5-hydroxydecanoate (7.9 g, 0.039 mol) and pyridine (manufactured by Junsei Chemical Co., Ltd., 31.6 g) obtained in Example 1 were put into a 100 mL three-necked flask, and stirred under ice water cooling at 0 A mixed solution of acetic anhydride (10.0 g, 0.098 mol) and 98% formic acid (manufactured by Junsei Chemical Co., Ltd., 4.6 g, 0.101 mol) was added dropwise at ~10°C / 30 minutes. After stirring overnight at room temperature, methanol (17.6 g, 0.55 mol) was added dropwise at 5 to 15° C. / 10 minutes under ice-cooling, and stirred at room temperature for 30 minutes. Pour into cold 10% hydrochloric acid (100mL), extract with ether (60mL×2 times), and wash the obtained ether layer with cold 10% hydrochloric acid (100mL), saturated aqueous sodium carbonate solution (100mL×2 times) and saturated saline ( 100mL) and washed in turn. After drying over anhydrous magnesium sulfate and concentrating und...
reference example 1
[0059] Reference Example 1: Synthesis of 5-acetoxydecanoic acid methyl ester
[0060] Methyl 5-hydroxydecanoate (10.1 g, 0.05 mol) and pyridine (manufactured by Junsei Chemical Co., Ltd., 40.3 g) obtained in Example 1 were put into a 100 mL three-necked flask, and stirred under ice water cooling with 5 Acetic anhydride (10.2 g, 0.10 mol) was added dropwise at ~8°C / 10 minutes. After stirring overnight at room temperature, methanol (18.0 g, 0.56 mol) was added dropwise at 5 to 15° C. / 10 minutes under ice-cooling, and stirred at room temperature for 30 minutes. Pour into cold 10% hydrochloric acid (100mL), extract with ether (60mL×2 times), and wash the obtained ether layer with cold 10% hydrochloric acid (100mL), saturated aqueous sodium carbonate solution (100mL×2 times) and saturated saline ( 100mL) and washed in turn. After drying over anhydrous magnesium sulfate and concentrating under reduced pressure, the resulting residue (12.0 g) was purified by distillation to obtain ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com