Method for producing perphosphoric acid by virtue of wet-process phosphoric acid purification
A wet-process phosphoric acid and superphosphoric acid technology, applied in chemical instruments and methods, inorganic chemistry, peroxides/peroxyhydrates/peroxyacids/superoxides/ozone oxides, etc. Efficient purification of wet-process phosphoric acid and other problems to achieve the effects of reduced production time, long time consumption and improved chromaticity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
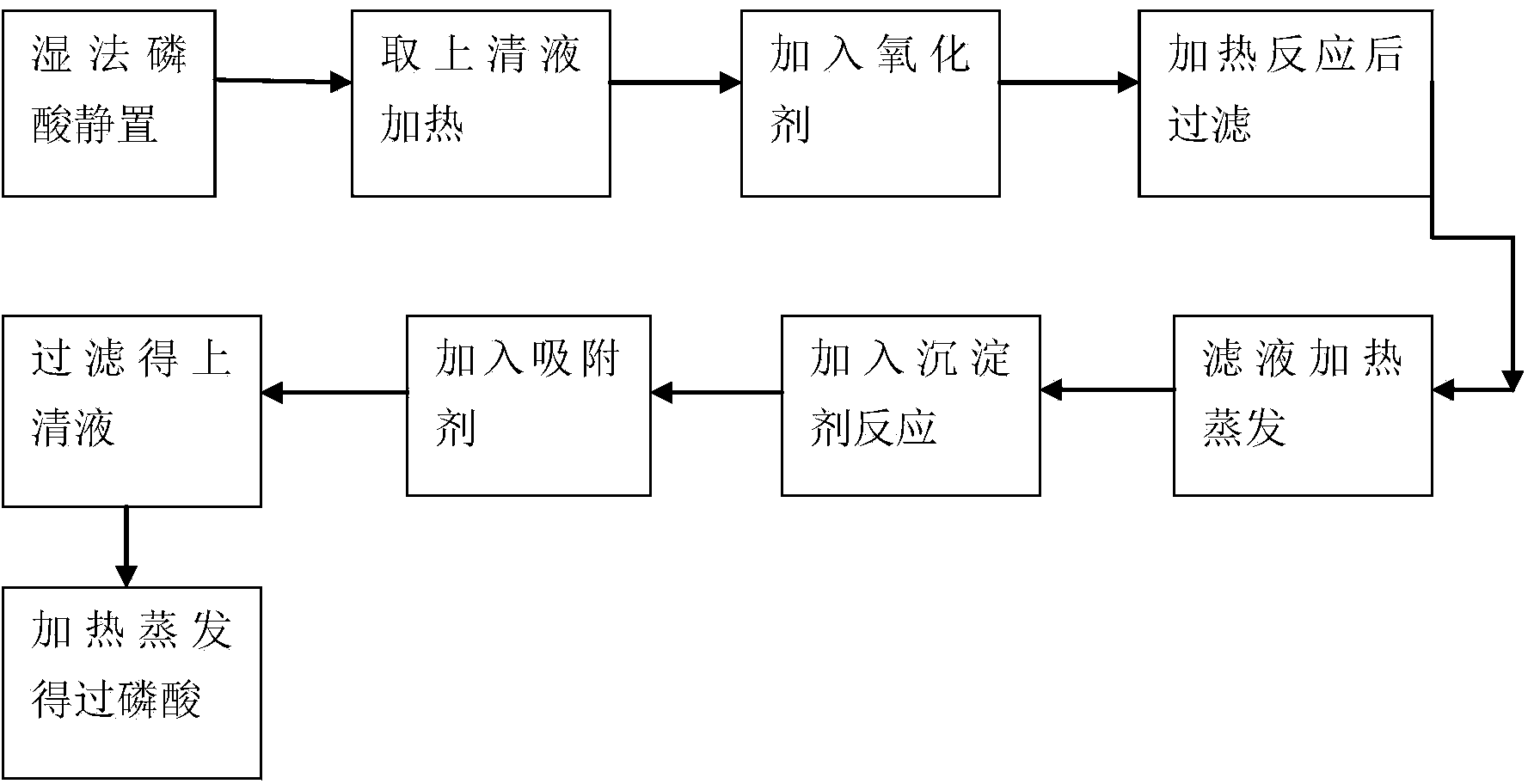
Image
Examples
specific Embodiment 1
[0019] 0.14g of oxidant ammonium persulfate was reacted with 200g of wet-process phosphoric acid (phosphorus pentoxide content: 35%) for 20 minutes at a temperature of 60°C, and then centrifuged for sedimentation, and the obtained filtrate was directly heated and evaporated to a concentration of 68% phosphorus pentoxide. % (150°C), cooled to 100°C, added 3.5g of precipitant diatomaceous earth, reacted for 60 minutes, cooled to 40°C, added 140g of ethanol for precipitation, and then added 0.14g of polyacrylamide containing polyacrylamide after precipitation solution. After stirring for 30 minutes, the purified phosphoric acid obtained at this time was colorless and transparent. This purified phosphoric acid is heated, evaporated and concentrated (with an additional rectification device to collect ethanol) to obtain a colorless and transparent superphosphoric acid with good fluidity. The polymerization rate was found to be 62% by chromatography.
[0020] By atomic absorption s...
specific Embodiment 2
[0021] 0.9g of oxidant potassium permanganate was reacted with 200g of wet-process phosphoric acid (content of phosphorus pentoxide: 45%) for 100 minutes at a temperature of 100°C, and then centrifuged for sedimentation, and the obtained filtrate was directly heated and evaporated to the concentration of phosphorus pentoxide: 55% (135°C), cooled to 100°C, added 0.9g precipitant diatomaceous earth, reacted for 30 minutes, cooled to 40°C, added 45g methanol to precipitate, added 1.8g activated carbon after precipitation, stirred for 60 minutes and filtered , the purified phosphoric acid obtained at this time is colorless and transparent. The purified phosphoric acid was heated, evaporated and concentrated (methanol was collected by an additional rectification device) to obtain a colorless and transparent superphosphoric acid with good fluidity. The polymerization rate was found to be 67% by chromatography.
[0022] By atomic absorption spectrophotometry, Fe% was 0.0846, Mg% was...
specific Embodiment 3
[0023] Under 85 ℃ of temperature conditions, 0.4g oxidant hydrogen peroxide, reacted with 200g wet process phosphoric acid (phosphorus pentoxide content: 40%) for 50 minutes, centrifugal sedimentation, the obtained filtrate is directly heated and evaporated to the concentration of phosphorus pentoxide to be 65% ( 150°C), cooled to 100°C and added 2.4g of precipitant diatomaceous earth, reacted for 30 minutes, cooled to 55°C, added 80g of n-propanol for precipitation, and then added 0.04g of adsorbent polyacrylamide and 0.4g of activated carbon after precipitation . After stirring for 10 minutes, the purified phosphoric acid obtained at this time was colorless and transparent. This purified phosphoric acid is heated, evaporated and concentrated (n-propanol is collected by an additional rectification device) to obtain a colorless and transparent superphosphoric acid with good fluidity. The polymerization rate was found to be 65% by chromatography.
[0024] By atomic absorption...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com