Adamantyl amino-acid ester compound synthesis method
An adamantane amino acid ester and a synthesis method technology are applied in the field of synthesizing adamantane amino acid ester compounds, can solve the problems of insufficient stability and high cost, and achieve the effects of mild reaction conditions, low cost and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
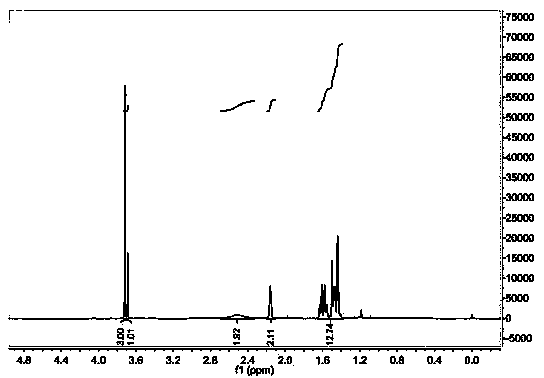
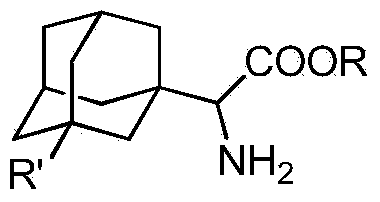
[0032] A kind of adamantyl amino ester compound, its structural formula is as follows:
[0033]
[0034] Wherein R is methyl, R' is OH.
[0035] The synthetic method of above-mentioned a kind of adamantyl amino ester compound specifically comprises the following steps:
[0036] (1) In a 50mL three-neck flask equipped with a thermometer, reflux condenser and vacuum plug, add 1.0g (4.2mmol) of compound 2, then add 21.0mL of solvent methanol and stir to dissolve completely, add 1.03g (8.4mmol) of compound 3 , after completely dissolving, add 0.09g (0.84mmol) triethylamine dropwise. After the addition is completed, the balloon is placed above the reflux condenser to isolate the air, and the reaction temperature is controlled at 60-65°C for 24 hours. Remove methyl alcohol, obtain imine compound crude product;
[0037] The structural formula of the compound 2 is as follows:
[0038] Wherein R is a methyl group, R' is a hydroxyl group, that is, compound 2 is (3-OH-1-adamantan...
Embodiment 2
[0049] A kind of adamantyl amino ester compound, its structural formula is as follows:
[0050]
[0051] Wherein R is methyl, R' is OH.
[0052] The synthetic method of above-mentioned a kind of adamantyl amino ester compound specifically comprises the following steps:
[0053](1) In a 50mL three-necked flask equipped with a thermometer, a reflux condenser and a vacuum plug, add 1.0g (4.2mmol) of compound 2, then add 21.0mL of solvent THF and stir to dissolve, add 1.03g (8.4mmol) of compound 3, After completely dissolving, add 0.09g (0.84mmol) triethylamine dropwise. After the addition is complete, put the balloon cover above the reflux condenser to isolate the air, and control the reaction temperature to 60-70°C for 24 hours. TLC detects that the basic reaction of the raw materials is complete. Rotary evaporation Remove tetrahydrofuran to obtain the imine compound crude product;
[0054] The structural formula of the compound 2 is as follows:
[0055] Wherein R is a m...
Embodiment 3
[0062] A kind of adamantyl amino ester compound, its structural formula is as follows:
[0063]
[0064] Wherein R is methyl, R' is OH.
[0065] The synthetic method of above-mentioned a kind of adamantyl amino ester compound specifically comprises the following steps:
[0066] (1) In a 50mL three-neck flask equipped with a thermometer, reflux condenser and vacuum plug, add 1.0g (4.2mmol) of compound 2, then add 21.0mL of solvent dichloromethane and stir to dissolve completely, add 1.03g (8.4mmol) After compound 3 was completely dissolved, 0.09g (0.84mmol) triethylamine was added dropwise. After the addition, the balloon was placed above the reflux condenser to isolate the air, and the reaction temperature was controlled at 30-40°C for 24 hours. TLC detected the raw material in the reaction. Residue, dichloromethane was removed by rotary evaporation to obtain the crude imine compound;
[0067] The structural formula of the compound 2 is as follows:
[0068] Wherein R i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com