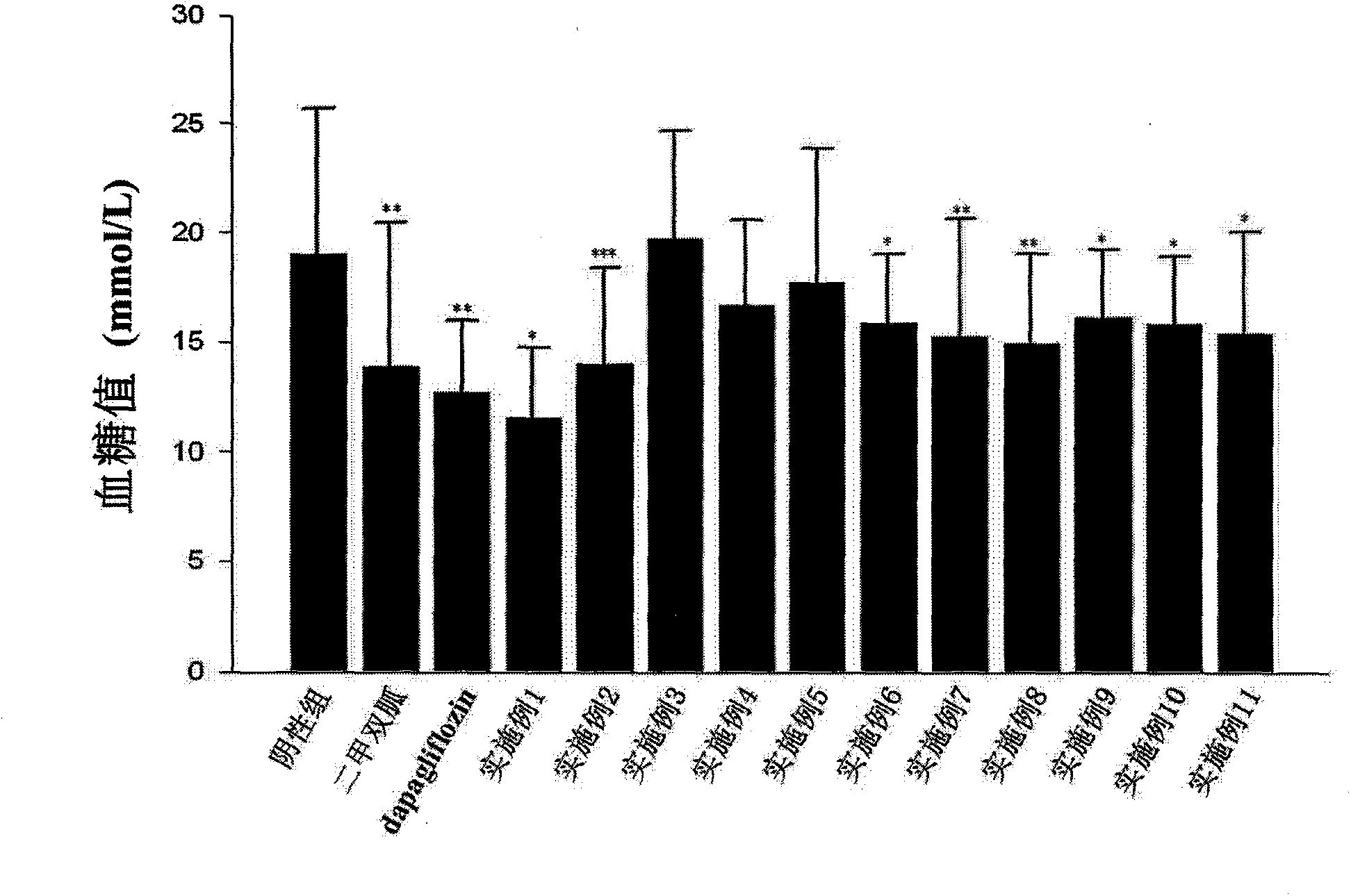
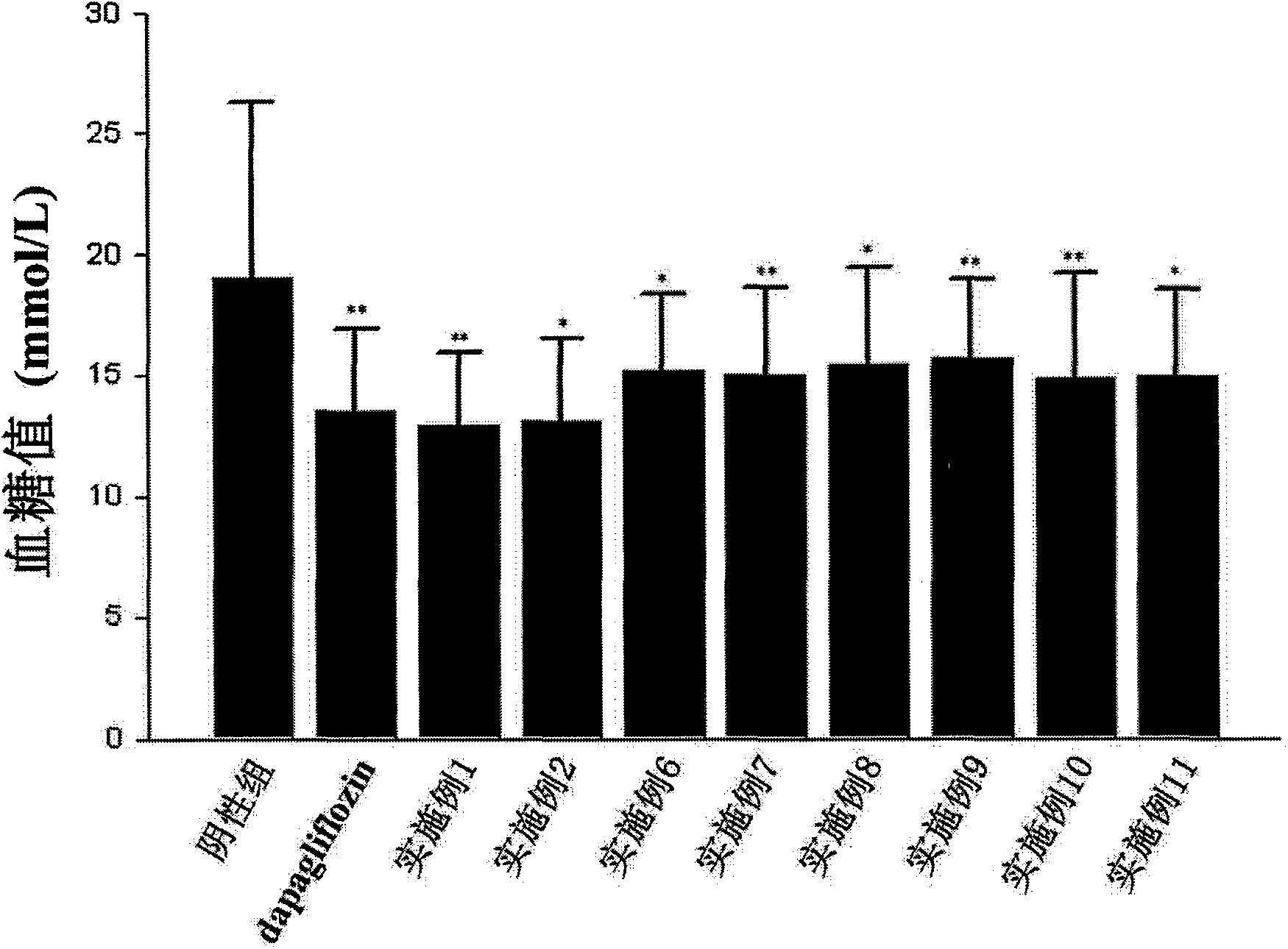
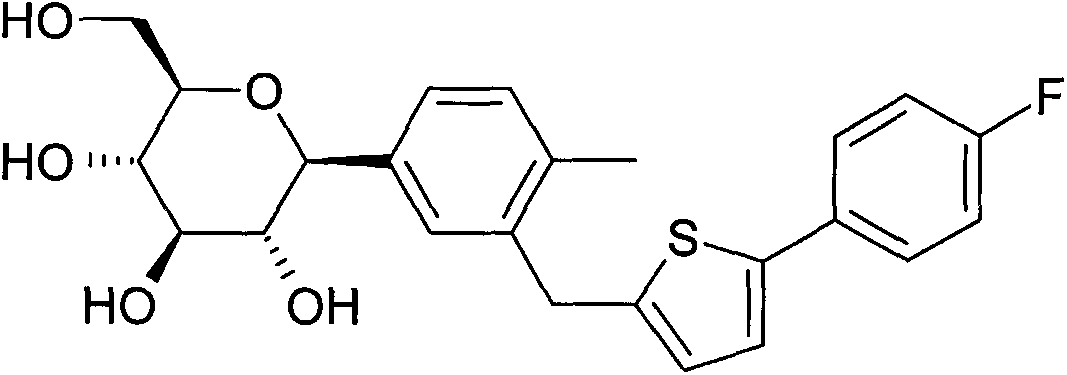
C-aryl glucoside derivatives, preparation method and application thereof
An alkyl compound technology, applied in the field of type 2 sodium-dependent glucose transporter inhibitors and its preparation, can solve the problems that tablets are difficult to store, easy to absorb moisture, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 6-O-benzoyl-1-{4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl}-β-D-glucopyranose
[0033]
[0034] A. Preparation of 2,3,4,6-tetra-O-trimethylsilyl-D-gluconolactone (III)
[0035]
[0036] Add 70g of D-glucono-1,5-lactone and 700ml of THF (re-distilled) into a 2L three-necked flask. Add 360g of N-methylmorpholine under stirring, cool to 5°C, then add 296g of trimethylchlorosilane, stir the slurry for 15 minutes, heat up to 35°C overnight.
[0037] The reactant was cooled to below 5°C, 1L of toluene was added, and then 1400ml of water was added to quench the reaction. Liquid separation, add 26g NaH 2 PO 4 , 520g of aqueous solution. The aqueous phase was separated, washed with water, brine, and washed with anhydrous Na 2 SO 4 Drying and concentration gave 200 g of an oil.
[0038] B. Preparation of 2-chloro-5-bromo-4'-ethoxydiphenylmethane
[0039]
[0040] 1. Preparation of 2-chloro-5-bromobenzoyl chloride
[0041]
[0042] Add 224g of 2-chloro-5-bromobe...
Embodiment 2
[0068] 6-O-ethylcarbonate-1-{4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl}-β-D-glucopyranose
[0069]
[0070] Preparation of 6-O-ethylcarbonate-1-{4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl}-1-deoxy-β-D-glucopyranose
[0071]
[0072] In a 250ml round bottom flask, add 5g of 1-C-(2-chloro-4'-ethoxydiphenylmethane-3-yl)-β-D-glucopyranose, 80ml of 2-methyltetrahydrofuran and 2g of diethyl carbonate Ester, stirred at 50°C for 24h. It was washed twice with saturated sodium bicarbonate solution, twice with water, and finally with brine, dried over anhydrous sodium sulfate, and concentrated. Separation by column chromatography (ethyl acetate / n-hexane = 1:2) gave a white foamy solid as 6-O-ethylcarbonate-1-{4-chloro-3-[(4-ethoxyphenyl )methyl]phenyl}-β-D-glucopyranose.
[0073] 1 HNMR (400MHz, CDCl 3 ): δ1.28(t, 3H), 1.39(t, 3H), 3.00(s, 4H), 3.40-3.61(m, 3H), 3.96-4.08(m, 5H), 4.16(q, 2H), 4.41(s, 2H), 6.80(d, 2H), 7.08(d, 2H), 7.20(d, 2H), 7.35(d, 1H)
Embodiment 3
[0075] 6-O-tert-butylcarbonate-1-{4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl}-β-D-glucopyranose
[0076]
[0077] Preparation of 6-O-tert-butylcarbonate-1-{4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl}-1-deoxy-β-D-glucopyranose
[0078]
[0079] Add 5 g of 1-C-(2-chloro-4′-ethoxydiphenylmethane-3-yl)-β-D-glucopyranose, 80 ml of 2-methyltetrahydrofuran and 2.5 g of chloroformic acid in a 250 ml round bottom flask Tert-butyl ester, stirred at 30°C for 24h. It was washed twice with saturated sodium bicarbonate solution, twice with water, and finally with brine, dried over anhydrous sodium sulfate, and concentrated. Separation by column chromatography (ethyl acetate / n-hexane = 1:2) gave a white foamy solid as 6-O-tert-butylcarbonate-1-{4-chloro-3-[(4-ethoxybenzene base)methyl]phenyl}-β-D-glucopyranose.
[0080] 1 HNMR (400MHz, CDCl 3 ): δ1.32(t, 3H), 1.38(s, 9H), 3.38-3.60(m, 6H), 3.96(s, 2H), 4.02-4.26(m, 4H), 4.41(m, 2H), 6.80(d, 2H), 7.08(d, 2H), 7.20(d, 2H), 7.35(d, 1H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com