High-drug loading capacity oxcarbazepine controlled-release granule and preparation method thereof
A technology of high drug loading and granules, which is applied in the field of medicine, can solve the problems of transportation, storage, inconvenient portability, enrichment of suspension particles in the suspension medium, and poor oral applicability, so as to improve portability, The effect of high drug loading and small batch-to-batch variation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
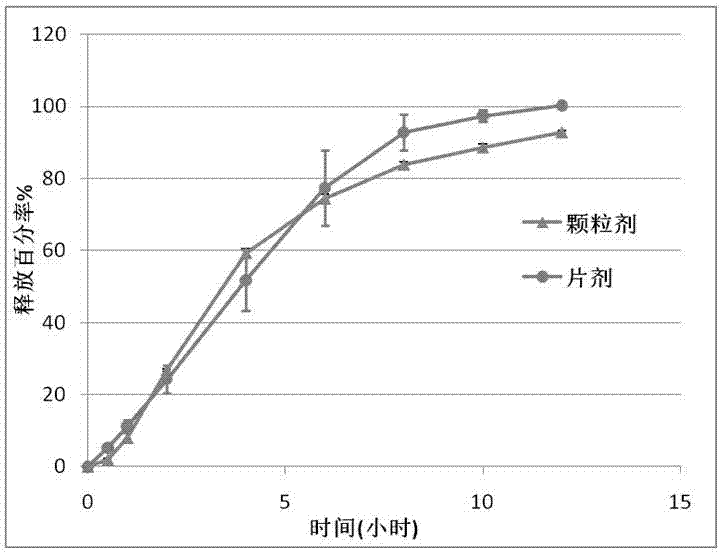
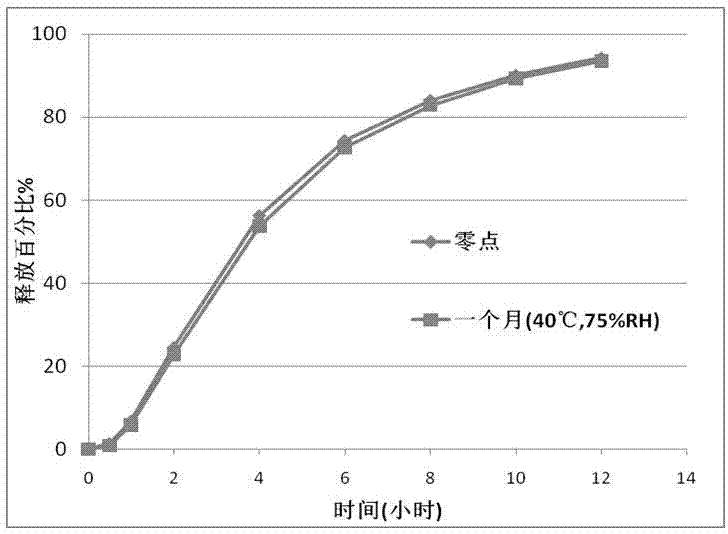
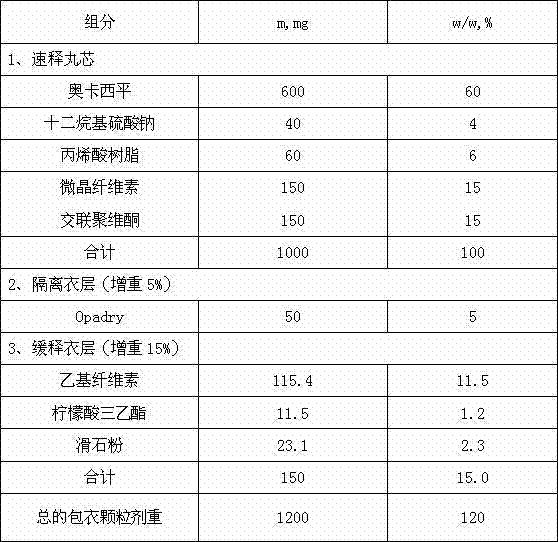
[0025] Example 1 Representative oxcarbazepine extended-release granules (immediate-release pellet cores prepared by extrusion spheronization, coated with Opadry I isolation coating suspension and acrylic resin extended-release coating suspension) are provided in Table 1 middle.
[0026] Table 1 Components of oxcarbazepine sustained-release granules
[0027]
[0028] Preparation steps of immediate-release pellets:
[0029] First, the poloxamer is formulated as a wetting agent with an appropriate amount of water. Weigh the rest of the raw and auxiliary materials in the prescribed amount, sieve and break the blocks, and place them in a wet granulator for dry mixing. Add an appropriate amount of wetting agent to it under stirring, and stir evenly to obtain a soft material; place the soft material in an extruder equipped with a 0.6mm aperture screen and extrude at a speed of 25rpm, and the strips pass through a spheronizer at 800rpm Spheronize for 3 minutes to obtain pellets ...
Embodiment 2
[0038] Example 2 Representative oxcarbazepine extended-release granules (immediate-release pellet cores prepared by hot-melt extrusion process, coated with Opadry I barrier coating suspension and acrylic resin extended-release coating suspension) are provided in Table 2 in.
[0039] Table 2 Components of oxcarbazepine sustained-release granules
[0040]
[0041] Preparation steps of immediate-release pellets:
[0042] The high-loaded oxcarbazepine immediate-release pellet core was prepared by hot-melt extrusion process, and the specific process was as follows:
[0043]1. Preparation of physical mixture: Weigh the raw and auxiliary materials of the prescription amount, sieve and break them, and dry mix them evenly in a mixer;
[0044] 2. Extrusion and granulation: set the temperature f of the conveying section, melting section, mixing section, and metering section of the hot-melt extruder to 40, 60, 60, and 40°C respectively. After preheating for 30 minutes, the above phys...
Embodiment 3
[0047] Example 3 Representative oxcarbazepine sustained-release granules (sodium lauryl sulfate was used instead of poloxamer, pellet cores were prepared by extrusion spheronization) are shown in Table 3.
[0048] Table 3 Components of oxcarbazepine sustained-release granules
[0049]
[0050] Preparation steps of immediate-release pellets:
[0051] Weigh the raw and auxiliary materials of the prescription amount, sieve and break the blocks, and place them in a wet granulator for dry mixing. at 300rpm
[0052] Add an appropriate amount of wetting agent to it under high-speed stirring, and stir evenly to obtain a soft material; place the soft material in an extruder equipped with a 0.6mm aperture screen and extrude at a speed of 35rpm, and the strips pass through a spheronizer at 1000rpm Spheronize for 3 minutes to obtain pellets with uniform particle size; dry the pellets at 60°C until the water content is less than 2%, and sieve the pellets with a size between 500-800 um...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com